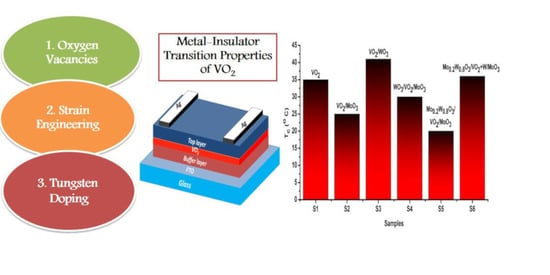
Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Thin Films
2.2. Characterization Techniques
3. Results and Discussion
3.1. Characterization of FTO Supported Oxide Thin Film
3.2. Effect of the Buffer Layer on the VO2 Properties
3.3. Anti-Reflective Top Layer Surface State
3.4. FTIR Spectroscopic, Optical Properties, and Urbach Tail
3.5. Electric Properties
| Structure | Preparation Method | Tunneling Effect | Phase Transition Temperature | Ref. |
|---|---|---|---|---|
| Cr-doped VO2 | Pulsed laser deposition | Doping | 34 °C | [57] |
| Mo-doped VO2 | DC sputtering | Doping | 63 °C | [58] |
| W-doped VO2 | Sol–gel | Doping | 36 °C | [47] |
| W-doped VO2 | DC sputtering | Doping | 37.4 °C | [59] |
| Al-doped VO2 | DC sputtering | Doping | 44.9 °C | [60] |
| V2O5/metal V/V2O5, V2O5/metal W/V2O5 multilayers | RF sputtering | Sandwich structure | 55 °C 48 °C | [61] |
| VO2 on MgF2 (110) | Oxide MBE method | Interfacial strain and oxygen vacancies | 69 °C | [62] |
| W-doped VO2 | Hydrothermal | Doping | 31.64 °C | [63] |
| VO2 | Sol–gel dip coating | - | 68 °C | [64] |
| Mo0.2W0.8O3/VO2/MoO3 | DC, RF Sputtering | Oxygen vacancy concentration, lattice strain, and W-doping effects | 20 °C | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, R.; Sardar, M.; Dhara, S. Origin of phase transition in VO2. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1942, p. 030003. [Google Scholar]
- Morin, F.J. Oxides which show a metal-to-insulator transition at the neel temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Luo, Y.; Yi, Z.; Zhang, J.; Liu, Z.; Yang, W.; Yu, Y.; Wu, X.; Wu, P. A switchable terahertz device combining ultra-wideband absorption and ultra-wideband complete reflection. Phys. Chem. Chem. Phys. 2022, 24, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, Y.; Luo, Y.; Yi, Z.; Zhang, J.; Liu, L.; Song, Q.; Wu, P.; Yu, Y.; Zhang, J. Terahertz perfect absorber based on flexible active switching of ultra-broadband and ultra-narrowband. Opt. Express 2021, 29, 42787. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, T.; Jiang, C.; Tang, B. Thermally switching between perfect absorber and asymmetric transmission in vanadium dioxide-assisted metamaterials. Opt. Express 2021, 29, 7666. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, H.; Zhang, Z.; Kang, L.; Chen, Z.; Du, J.; Kanehira, M.; Cao, C. Nanoceramic VO2 thermochromic smart glass: A review on progress in solution processing. Nano Energy 2012, 1, 221–246. [Google Scholar] [CrossRef]
- Hanlon, T.J.; Coath, J.A.; Richardson, M.A. Molybdenum-doped vanadium dioxide coatings on glass produced by the aqueous sol-gel method. Thin Solid Films 2003, 436, 269–272. [Google Scholar] [CrossRef]
- Strelcov, E.; Tselev, A.; Ivanov, I.; Budai, J.D.; Zhang, J.; Tischler, J.Z.; Kravchenko, I.; Kalinin, S.V.; Kolmakov, A. Doping-based stabilization of the M2 phase in free-standing VO2 nanostructures at room temperature. Nano Lett. 2012, 12, 6198–6205. [Google Scholar] [CrossRef]
- Bergerudm, A.J. Phase Stability and Transformations in Vanadium Oxide Nanocrystals. Ph.D. Thesis, University of California, Berkeley, CA, USA.
- Ganduglia-Pirovano, M.V.; Hofmann, A.; Sauer, J. Oxygen vacancies in transition metal and rare earth oxides: Current state of understanding and remaining challenges. Surf. Sci. Rep. 2007, 62, 219–270. [Google Scholar] [CrossRef]
- Singh, S.; Abtew, T.A.; Horrocks, G.; Kilcoyne, C.; Marley, P.M.; Stabile, A.A.; Banerjee, S.; Zhang, P.; Sambandamurthy, G. Selective electrochemical reactivity of rutile VO2 towards the suppression of metal-insulator transition. Phys. Rev. B 2016, 93, 125132. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Wong, L.M.; Wang, S.J.; Tang, S.H.; Zhang, X.H. Effect of oxygen stoichiometry on the insulator-metal phase transition in vanadium oxide thin films studied using optical pump-terahertz probe spectroscopy. Appl. Phys. Lett. 2013, 103, 151908. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Li, J.; Jin, H.; Rehman, F.; Chen, P.; Jiang, Y.; Chen, C.; Cao, M.; Zhao, Y. Evolution of Structural and Electrical Properties of Oxygen-Deficient VO2 under Low Temperature Heating Process. ACS Appl. Mater. Interfaces 2017, 9, 27135–27141. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, Y.; Shi, S.; Liu, B.; Luo, H.; Gao, Y. First-principles study of the effect of oxygen vacancy and strain on the phase transition temperature of VO2. RSC Adv. 2016, 6, 86872–86879. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Shaban, M.; Eker, Y.R.; Yilmaz, M. Efficient MoWO3/VO2/MoS2/Si UV Schottky photodetectors; MoS2 optimization and monoclinic VO2 surface modifications. Sci. Rep. 2020, 10, 15926. [Google Scholar] [CrossRef] [PubMed]
- Appavoo, K.; Lei, D.Y.; Sonnefraud, Y.; Wang, B.; Pantelides, S.T.; Maier, S.A.; Haglund, R.F. Role of defects in the phase transition of VO2 nanoparticles probed by plasmon resonance spectroscopy. Nano Lett. 2012, 12, 780–786. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast response of CO2 room temperature gas sensor based on Mixed-Valence Phases in Molybdenum and Tungsten Oxide nanostructured thin films. Ceram. Int. 2020, 46, 9839–9853. [Google Scholar] [CrossRef]
- Currie, M.; Mastro, M.A.; Wheeler, V.D. Atomic layer deposition of vanadium dioxide and a temperature-dependent optical model. J. Vis. Exp. 2018, 2018, e57103. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ji, S.; Gao, Y.; Luo, H.; Kanehira, M. Core-shell VO2 @TiO2 nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings. Sci. Rep. 2013, 3, 1370. [Google Scholar] [CrossRef]
- Makarevich, A.M.; Sadykov, I.I.; Sharovarov, D.I.; Amelichev, V.A.; Adamenkov, A.A.; Tsymbarenko, D.M.; Plokhih, A.V.; Esaulkov, M.N.; Solyankin, P.M.; Kaul, A.R. Chemical synthesis of high quality epitaxial vanadium dioxide films with sharp electrical and optical switch properties. J. Mater. Chem. C 2015, 3, 9197–9205. [Google Scholar] [CrossRef]
- Chen, X.B. Assignment of the Raman modes of VO2 in the monoclinic insulating phase. J. Korean Phys. Soc. 2011, 58, 100–104. [Google Scholar] [CrossRef]
- Mohamed, B.; Ali, M. VO2 İnce Film İçeren İki Boyutlu ve Çok Katmanlı Yapılardaki Arayüzey Gerilmelerinin Termokromik ve Fotodetektör Performanslara Etkisi. Master’s Thesis, University of Necmettin Erbakan, Konya, Turkey, August 2020. [Google Scholar]
- Nagyné-Kovács, T.; Studnicka, L.; Lukács, I.E.; László, K.; Pasierb, P.; Szilágyi, I.M.; Pokol, G. Hydrothermal synthesis and gas sensing of monoclinic MoO3 nanosheets. Nanomaterials 2020, 10, 891. [Google Scholar] [CrossRef]
- Martín-Ramos, P.; Fernández-Coppel, I.A.; Avella, M.; Martín-Gil, J. α-MoO3 crystals with a multilayer stack structure obtained by annealing from a lamellar MoS2/g-C3N4 nanohybrid. Nanomaterials 2018, 8, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, T.; Gesheva, K.A.; Szekeres, A. Structure and optical properties of CVD molybdenum oxide films for electrochromic application. J. Solid State Electrochem. 2002, 7, 21–24. [Google Scholar] [CrossRef]
- Kee, C.W. Assignment of O-O and Mo=O Stretching Frequencies of Molybdenum/Tungsten Complexes Revisited. J. Chem. 2015, 2015, 439270. [Google Scholar] [CrossRef] [Green Version]
- Klinbumrung, A.; Thongtem, T.; Thongtem, S. Characterization of orthorhombic α-MoO3 microplates produced by a microwave plasma process. J. Nanomater. 2012, 2012, 930763. [Google Scholar] [CrossRef] [Green Version]
- Haque, F.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Yin, Y.; Yi, Z.; Wang, Y.; Mahmood, N.; Pillai, N.; Syed, N.; et al. Ordered intracrystalline pores in planar molybdenum oxide for enhanced alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 257–268. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Xia, Q.; Tian, L.; Chen, X. Ultrathin tungsten oxide nanowires: Oleylamine assisted nonhydrolytic growth, oxygen vacancies and good photocatalytic properties. RSC Adv. 2015, 5, 77423–77428. [Google Scholar] [CrossRef]
- Díaz-Reyes, J.; Castillo-Ojeda, R.; Galván-Arellano, M.; Zaca-Moran, O. Characterization of WO3 thin films grown on silicon by HFMOD. Adv. Condens. Matter Phys. 2013, 2013, 591787. [Google Scholar] [CrossRef] [Green Version]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of oxygen vacancies in vanadium oxide and oxygen functional groups in graphene oxide for room temperature CO2 gas sensors. Sens. Actuators A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Grey, I.E.; Wilson, N.C. Titanium vacancy defects in sol-gel prepared anatase. J. Solid State Chem. 2007, 180, 670–678. [Google Scholar] [CrossRef]
- Jørgensen, J.E.; Mosegaard, L.; Thomsen, L.E.; Jensen, T.R.; Hanson, J.C. Formation of γ-Fe2O3 nanoparticles and vacancy ordering: An in situ X-ray powder diffraction study. J. Solid State Chem. 2007, 180, 180–185. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, N.; Huang, S.; Zhou, G.; Hou, J.; Cao, Z.; Zhu, H. Structural studies of TiC1−xOx solid solution by Rietveld refinement and first-principles calculations. J. Solid State Chem. 2013, 204, 1–8. [Google Scholar] [CrossRef]
- Ferri, E.A.V.; Sczancoski, J.C.; Cavalcante, L.S.; Paris, E.C.; Espinosa, J.W.M.; de Figueiredo, A.T.; Pizani, P.S.; Mastelaro, V.R.; Varela, J.A.; Longo, E. Photoluminescence behavior in MgTiO3 powders with vacancy/distorted clusters and octahedral tilting. Mater. Chem. Phys. 2009, 117, 192–198. [Google Scholar] [CrossRef]
- Somogyvári, Z.; Sváb, E.; Mészáros, G.; Krezhov, K.; Nedkov, I.; Sajó, I.; Bourée, F. Vacancy ordering in nanosized maghemite from neutron and X-ray powder diffraction. Appl. Phys. A Mater. Sci. Process. 2002, 74, s1077–s1079. [Google Scholar] [CrossRef]
- Fan, S.; Fan, L.; Li, Q.; Liu, J.; Ye, B. The identification of defect structures for oxygen pressure dependent VO2 crystal films. Appl. Surf. Sci. 2014, 321, 464–468. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zhang, G.; Jiang, J. Insight into Electronic and Structural Reorganizations for Defect-Induced VO2 Metal-Insulator Transition. J. Phys. Chem. Lett. 2017, 8, 3129–3132. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Preparation of orthorhombic WO3 thin films and their crystal quality-dependent dye photodegradation ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Patterson, B.R. Stereological analysis of Zener pinning. Acta Mater. 1996, 44, 4327–4335. [Google Scholar] [CrossRef]
- Al Mohammad, A.; Gillet, M. Phase transformations in WO3 thin films during annealing. Thin Solid Films 2002, 408, 302–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, W.; Chen, W.; Zheng, Y. Recent progress on vanadium dioxide nanostructures and devices: Fabrication, properties, applications and perspectives. Nanomaterials 2021, 11, 338. [Google Scholar] [CrossRef]
- Ding, Z.; Cui, Y.; Wan, D.; Luo, H.; Gao, Y. High-performance thermal sensitive VO2(B) thin films prepared by sputtering with TiO2(A) buffer layer and first-principles calculations study. RSC Adv. 2017, 7, 29496–29504. [Google Scholar] [CrossRef] [Green Version]
- Rosevear, W.H.; Paul, W. Hall effect in VO2 near the semiconductor-to-metal transition. Phys. Rev. B 1973, 7, 2109–2111. [Google Scholar] [CrossRef]
- Wang, S.; Malyshev, O.B.; Valizadeh, R.; Seddon, E.A.; Cropper, M.D. The secondary electron yield from transition metals. In Proceedings of the IPAC 2014 5th International Particle Accelerator Conference, Dresden, Germany, 16 June 2013–20 June 2014; pp. 2403–2405. [Google Scholar]
- Zakharova, G.S.; Podval’Naya, N.V.; Kuznetsov, M.V. XPS study of nanorods of doped vanadium oxide Mx V2O5 · nH2O (M = Na, K, Rb, Cs). Russ. J. Inorg. Chem. 2011, 56, 267–272. [Google Scholar] [CrossRef]
- Pan, G.; Yin, J.; Ji, K.; Li, X.; Cheng, X.; Jin, H.; Liu, J. Synthesis and thermochromic property studies on W doped VO2 films fabricated by sol-gel method. Sci. Rep. 2017, 7, 6132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.Y.; Li, M.; Pan, S.S.; Zhang, Y.X.; Li, G.H. Synthesis and thermal stability of W-doped VO2 nanocrystals. Mater. Res. Bull. 2011, 46, 2100–2104. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Z.; Xu, X.; Wang, T.; Jiang, Y. Electrical and optical properties of nanostructured VOX thin films prepared by direct current magnetron reactive sputtering and post-annealing in oxygen. Thin Solid Films 2011, 519, 6203–6207. [Google Scholar] [CrossRef]
- Nam, S.P.; Noh, H.J.; Lee, S.G.; Lee, Y.H. Electrical properties of vanadium tungsten oxide thin films. Mater. Res. Bull. 2010, 45, 291–294. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Stanescu, R.; Cozar, I.B.; Ardelean, I. Structural investigation of new vanadium-bismuth-phosphate glasses by IR and ESR spectroscopy. J. Mol. Struct. 2013, 1044, 323–327. [Google Scholar] [CrossRef]
- Yoffe, A.D. Theory of Defects in Solids: Electronic Structure Defects in Insulators and Semiconductors. Phys. Bull. 1986, 37, 266–267. [Google Scholar] [CrossRef]
- Tuo, S.; Cattin, L.; Essaidi, H.; Peres, L.; Louarn, G.; El Jouad, Z.; Hssein, M.; Touihri, S.; Yapi Abbe, S.; Torchio, P.; et al. Stabilisation of the electrical and optical properties of dielectric/Cu/dielectric structures through the use of efficient dielectric and Cu:Ni alloy. J. Alloys Compd. 2017, 729, 109–116. [Google Scholar] [CrossRef]
- Kachi, S.; Takada, T.; Kosuge, K. Electrical Conductivity of Vanadium Oxides. J. Phys. Soc. Jpn. 1963, 18, 1839–1840. [Google Scholar] [CrossRef]
- Gurunatha, K.L.; Sathasivam, S.; Li, J.; Portnoi, M.; Parkin, I.P.; Papakonstantinou, I. Combined Effect of Temperature Induced Strain and Oxygen Vacancy on Metal-Insulator Transition of VO2 Colloidal Particles. Adv. Funct. Mater. 2020, 30, 2005311. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Wang, F.; Zhang, Q.; Zhu, L.; Meng, Q.; Wang, B.; Zhang, Z.; Zou, C. Revealing the role of oxygen vacancies on the phase transition of VO2 film from the optical-constant measurements. RSC Adv. 2018, 8, 19151–19156. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, A.O.; Mansouri, S.; Margot, J.; Chaker, M. Tuning VO2 phase stability by a combined effect of Cr doping and oxygen pressure. Appl. Surf. Sci. 2022, 571, 151267. [Google Scholar] [CrossRef]
- Batista, C.; Ribeiro, R.M.; Teixeira, V. Synthesis and characterization of VO2-based thermochromic thin films for energy-efficient windows. Nanoscale Res. Lett. 2011, 6, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Wu, Z.; Ji, C.; Dai, J.; Xiang, Z.; Wang, D.; Dong, X.; Jiang, Y. Improvement of phase transition properties of magnetron sputtered W-doped VO2 films by post-annealing approach. J. Mater. Sci. Mater. Electron. 2020, 31, 4150–4160. [Google Scholar] [CrossRef]
- Ji, C.; Wu, Z.; Wu, X.; Wang, J.; Gou, J.; Huang, Z.; Zhou, H.; Yao, W.; Jiang, Y. Al-doped VO2 films as smart window coatings: Reduced phase transition temperature and improved thermochromic performance. Sol. Energy Mater. Sol. Cells 2018, 176, 174–180. [Google Scholar] [CrossRef]
- Liu, H.; Wan, D.; Ishaq, A.; Chen, L.; Guo, B.; Shi, S.; Luo, H.; Gao, Y. Sputtering Deposition of Sandwich-Structured V2O5/Metal (V, W)/V2O5 Multilayers for the Preparation of High-Performance Thermally Sensitive VO2 Thin Films with Selectivity of VO2 (B) and VO2 (M) Polymorph. ACS Appl. Mater. Interfaces 2016, 8, 7884–7890. [Google Scholar] [CrossRef]
- Fan, L.L.; Chen, S.; Liao, G.M.; Chen, Y.L.; Ren, H.; Zou, C.W. Comprehensive studies of interfacial strain and oxygen vacancy on metal-insulator transition of VO2 film. J. Phys. Condens. Matter 2016, 28, 255002. [Google Scholar] [CrossRef]
- Barra, H.M.; Chen, S.K.; Tamchek, N.; Talib, Z.A.; Lee, O.J.; Tan, K.B. Phase, Microstructure, Thermochromic, and Thermophysical Analyses of Hydrothermally Synthesized W-Doped VO2 Nanopowder. Adv. Mater. Sci. Eng. 2021, 2021, 8582274. [Google Scholar] [CrossRef]
- Chotirat, L.; Niyomwas, S.; Wongpisan, W.; Supothina, S. Low-Temperature Synthesis of Vanadium Dioxide Thin Films by Sol-Gel Dip Coating Method. J. Nanotechnol. 2021, 2021, 4868152. [Google Scholar] [CrossRef]
- Görmez, A.E.; Basyooni, M.A.; Zaki, S.E.; Eker, Y.R.; Sönmez, E.; Yılmaz, M. Effect of in-/ex-situ annealing temperture on the optical, structural and gas sensing dynamics of CdS nanostructured thin films. Superlattices Microstruct. 2020, 142, 106536. [Google Scholar] [CrossRef]
- Basyooni, M.A.M.A.; Shaban, M.; El Sayed, A.M.A.M. Enhanced Gas Sensing Properties of Spin-coated Na-doped ZnO Nanostructured Films. Sci. Rep. 2017, 7, 41716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shportko, K.V. Disorder and compositional dependences in Urbach-Martienssen tails in amorphous (GeTe)x(Sb2Te3)1−x alloys. Sci. Rep. 2019, 9, 6030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonalde, I.; Medina, E.; Rodríguez, M.; Wasim, S.M.; Marín, G.; Rincón, C.; Rincón, A.; Torres, C. Urbach tail, disorder, and localized modes in ternary semiconductors. Phys. Rev. B 2004, 69, 195201. [Google Scholar] [CrossRef]
- Mishra, V.; Sagdeo, A.; Warshi, K.; Rai, H.M.; Saxena, S.; Kumar, R.; Sagdeo, P. Metastable behavior of Urbach tail states in BaTiO3 across phase transition. arXiv 2016, arXiv:1612.06756. Available online: https://arxiv.org/abs/1612.06756v1 (accessed on 1 March 2022).








| Notation | Structure | Layer | Power of Mo (W) RF1 | Power of W (W) RF2 | Power of V (W) DC | Ar Flow (sccm) | O2 Flow (sccm) | Time (min) |
|---|---|---|---|---|---|---|---|---|
| S1 | VO2 | 1st layer: VO2 | - | - | 190 | 41 | 2.2 | 7.5 |
| S2 | VO2/MoO3 | 1st layer: MoO3 | 137 | - | - | 37.1 | 12.1 | 16.7 |
| 2nd layer: VO2 | - | - | 190 | 41 | 2.2 | 7.5 | ||
| S3 | VO2/WO3 | 1st layer: WO3 | - | 137 | - | 37.1 | 12.1 | 7.5 |
| 2nd layer: VO2 | - | - | 190 | 41 | 2.2 | 7.5 | ||
| S4 | WO3/VO2/MoO3 | 1st layer: MoO3 | 137 | - | - | 37.1 | 12.1 | 16.7 |
| 2nd layer: VO2 | - | - | 190 | 41 | 2.2 | 7.5 | ||
| 3rd layer: WO3 | - | 137 | - | 37.1 | 12.1 | 7.5 | ||
| S5 | Mo0.2W0.8O3/VO2/MoO3 | 1st layer: MoO3 | 137 | - | - | 37.1 | 12.1 | 16.7 |
| 2nd layer: VO2 | - | - | 190 | 41 | 2.2 | 7.5 | ||
| 3rd layer: Mo0.2W0.8O3 | 27 | 110 | - | 37.1 | 12.1 | 7.5 | ||
| S6 | Mo0.2W0.8O3/VO2 + W/MoO3 | 1st layer: MoO3 | 137 | - | - | 37.1 | 12.1 | 16.7 |
| 2nd layer: VO2 + W | - | 10 | 190 | 41 | 2.2 | 7.5 | ||
| 3rd layer: Mo0.2W0.8O3 | 27 | 110 | - | 37.1 | 12.1 | 7.5 |
| Sample | 2θ (°) | β (°) | D (nm) | δ × 10−3 (nm−2) | ε × 10−3 |
|---|---|---|---|---|---|
| V–O | 27.936 | 0.399 | 20.5 | 2.4 | 7.01 |
| Mo–O | 23.254 | 0.479 | 17.0 | 3.5 | 10.18 |
| Mo–W–O | 23.309 | 1.359 | 6.0 | 28.1 | 28.76 |
| W–O | 23.222 | 1.358 | 5.5 | 32.9 | 28.84 |
| Sample | Mean Grain Area (μm2) | Ra (nm) | Rq (nm) | Rpv (nm) |
|---|---|---|---|---|
| S1 | 9.41 × 10−4 | 0.9 | 1.2 | 6.8 |
| S2 | 1.183 × 10−3 | 12.2 | 14.1 | 56.1 |
| S3 | 1.774 × 10−3 | 6.0 | 7.4 | 33.5 |
| S4 | 1.056 × 10−3 | 10.1 | 12.1 | 49.9 |
| S5 | 1.526 × 10−3 | 5.7 | 6.9 | 28.1 |
| S6 | 9.06 × 10−4 | 13.8 | 16.1 | 63.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basyooni, M.A.; Al-Dossari, M.; Zaki, S.E.; Eker, Y.R.; Yilmaz, M.; Shaban, M. Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping. Nanomaterials 2022, 12, 1470. https://doi.org/10.3390/nano12091470
Basyooni MA, Al-Dossari M, Zaki SE, Eker YR, Yilmaz M, Shaban M. Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping. Nanomaterials. 2022; 12(9):1470. https://doi.org/10.3390/nano12091470
Chicago/Turabian StyleBasyooni, Mohamed A., Mawaheb Al-Dossari, Shrouk E. Zaki, Yasin Ramazan Eker, Mucahit Yilmaz, and Mohamed Shaban. 2022. "Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping" Nanomaterials 12, no. 9: 1470. https://doi.org/10.3390/nano12091470
APA StyleBasyooni, M. A., Al-Dossari, M., Zaki, S. E., Eker, Y. R., Yilmaz, M., & Shaban, M. (2022). Tuning the Metal–Insulator Transition Properties of VO2 Thin Films with the Synergetic Combination of Oxygen Vacancies, Strain Engineering, and Tungsten Doping. Nanomaterials, 12(9), 1470. https://doi.org/10.3390/nano12091470