Multifunctional Biomimetic Composite Coating with Antireflection, Self-Cleaning and Mechanical Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the BCC
2.2.1. SiO2/PDMS/Matte Polyurethane Dispersion
2.2.2. The Pretreatment Process of the Aluminum Alloy Surface
2.3. Characterization
3. Results and Discussion
3.1. Morphologies and Composition Analysis of BCC
3.2. Antireflective and Self-Cleaning Properties of BCC
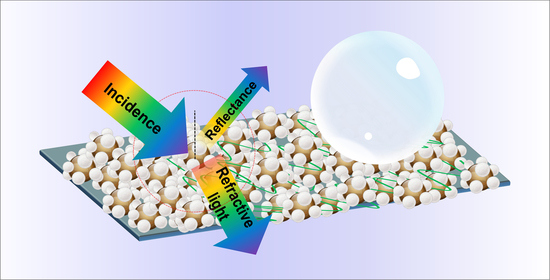
3.3. Antireflective and Self-Cleaning Mechanism
3.4. Mechanical Durability
3.5. Stability Test of BCC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswas, D.; Chundi, N.; Atchuta, S.R.; Phani Kumar, K.K.; Shiva Prasad, M.; Sakthivel, S. Fabrication of Omnidirectional Broadband Dual-Functional Coating with High Optical and Self-Cleaning Properties for Photovoltaic Application. Sol. Energy 2022, 246, 36–44. [Google Scholar] [CrossRef]
- Tian, Z.; Lei, Z.; Chen, X.; Chen, Y.; Zhang, L.C.; Bi, J.; Liang, J. Nanosecond Pulsed Fiber Laser Cleaning of Natural Marine Micro-Biofoulings from the Surface of Aluminum Alloy. J. Clean. Prod. 2020, 244, 118724. [Google Scholar] [CrossRef]
- Ghosh, M.; Rao, G.M. Superhydrophobic Vertically Aligned Treelike Carbon Nanostructures. Phys. Rev. Appl. 2019, 11, 034011. [Google Scholar] [CrossRef]
- Wang, K.; Yu, S.R.; Li, W.; Song, Y.J.; Gong, P.; Zhang, M.S.; Li, H.S.; Sun, D.J.; Yang, X.Z.; Wang, X.W. Superhydrophobic and Photocatalytic Synergistic Self-Cleaning ZnS Coating. Appl. Surf. Sci. 2022, 595, 153565. [Google Scholar] [CrossRef]
- Xia, X.J.; Liu, J.; Liu, Y.; Lei, Z.J.; Han, Y.T.; Zheng, Z.P.; Yin, J. Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings 2023, 13, 224. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Jiang, Y.; Sun, H.; Liu, T.; Li, G.; Yu, W.; Meng, C.; Guo, S. Bioinspired Flexible, Breathable, Waterproof and Self-Cleaning Iontronic Tactile Sensors for Special Underwater Sensing Applications. Nano Energy 2023, 110, 108367. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Yu, X.; Xu, L.; Wang, D.; Lu, X.; Zhang, A. Superhydrophobic MXene Coating with Biomimetic Structure for Self-Healing Photothermal Deicing and Photoelectric Detector. ACS Appl. Mater. Interfaces 2022, 14, 53298–53313. [Google Scholar] [CrossRef]
- Kang, S.M.; Choi, J.S.; Hong, J.; Lee, O.H.; Kang, I. Clearwing-Inspired Invisible Thin Membrane. Adv. Opt. Mater. 2023, 11, 2202825. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Li, Y.; Huang, L.; Tang, Y. Fluorine-Free, Highly Durable Waterproof and Breathable Fibrous Membrane with Self-Clean Performance. Nanomaterials 2023, 13, 516. [Google Scholar] [CrossRef]
- Zhao, S.; Du, H.; Ma, Z.; Xiao, G.; Liu, J.; Jiang, Y.; Hu, S.; Zhao, H.; Wen, C.; Ren, L. Efficient Fabrication of Ternary Coupling Biomimetic Superhydrophobic Surfaces with Superior Performance of Anti-Wetting and Self-Cleaning by a Simple Two-Step Method. Mater. Des. 2022, 223, 111145. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L.; Jiang, S.; Chen, L.; Sang, S.; Zhang, H. A Photocatalytic Degradation Self-Cleaning Composite Membrane for Oil-Water Separation Inspired by Light-Trapping Effect of Moth-Eye. J. Membr. Sci. 2023, 669, 121337. [Google Scholar] [CrossRef]
- Jacobo-Martín, A.; Hernández, J.J.; Solano, E.; Monclús, M.A.; Carlos Martínez, J.; Fernandes, D.F.; Pedraz, P.; Molina-Aldareguia, J.M.; Kubart, T.; Rodríguez, I. Resilient Moth-Eye Nanoimprinted Antireflective and Self-Cleaning TiO2 Sputter-Coated PMMA Films. Appl. Surf. Sci. 2022, 585, 152653. [Google Scholar] [CrossRef]
- Ma, Y.; Gou, T.; Feng, C.; Li, J.; Li, Y.; Huang, J.; Li, R.; Zhang, Z.; Gao, X. Facile Fabrication of Biomimetic Films with the Microdome and Tapered Nanonipple Hierarchical Structure Possessing High Haze, High Transmittance, Anti-Fouling and Moisture Self-Cleaning Functions. Chem. Eng. J. 2021, 404, 127101. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Yan, C.; Li, J.; Zhou, L.; Yin, X.; He, Y.; Zhao, Y.; Liu, M. A Bioinspired Strategy to Construct Dual-Superlyophobic PPMB Membrane for Switchable Oil/Water Separation. J. Membr. Sci. 2023, 665, 121128. [Google Scholar] [CrossRef]
- Chien, C.Y.; Chen, Y.H.; Chen, R.Y.; Yang, H. Dragonfly-Wing-Inspired Inclined Irregular Conical Structures for Broadband Omnidirectional Antireflection Coatings. ACS Appl. Nano Mater. 2020, 3, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wan, Y.; Qin, Z.; Xu, Q.; Min, Y. Fabrication and Corrosion Resistance of a Graphene-Tin Oxide Composite Film on Aluminium Alloy 6061. Corros. Sci. 2018, 130, 85–94. [Google Scholar] [CrossRef]
- Parra-Barranco, J.; Lopez-Santos, C.; Sánchez-Valencia, J.R.; Borras, A.; Gonzalez-Elipe, A.R.; Barranco, A. Mechanically Switchable Wetting Petal Effect in Self-Patterned Nanocolumnar Films on Poly(dimethylsiloxane). Nanomaterials 2021, 11, 2566. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Z.; Zhang, H.; Tian, S.; Li, X.; Fan, H.; Fu, S. Superhydrophobic and Deacidified Cellulose/CaCO3-Derived Granular Coating toward Historic Paper Preservation. Int. J. Biol. Macromol. 2022, 207, 232–241. [Google Scholar] [CrossRef]
- Qiu, R.; Li, Z.; Wu, Z. Enhanced Anti-Icing and Anti-Corrosion Properties of Abrasion-Resistant Superhydrophobic Surfaces Based on Al Alloys. Mater. Res. Express 2019, 6, 045059. [Google Scholar] [CrossRef]
- Guo, X.; Guo, R.; Fang, M.; Wang, N.; Liu, W.; Pei, H.; Liu, N.; Mo, Z. A Novel Composite Protective Coating with UV and Corrosion Resistance: Load Floating and Self-Cleaning Performance. Ceram. Int. 2022, 48, 17308–17318. [Google Scholar] [CrossRef]
- Sun, H.; Lei, F.; Li, T.; Han, H.; Li, B.; Li, D.; Sun, D. Facile Fabrication of Novel Multifunctional Lubricant-Infused Surfaces with Exceptional Tribological and Anticorrosive Properties. ACS Appl. Mater. Interfaces 2021, 13, 6678–6687. [Google Scholar] [CrossRef] [PubMed]
- Ley, J.R.; Kwon, Y.W.; Park, C.; Menon, S.K. Corrosion of Femtosecond Laser Surface Textured Aluminium Alloy. Corros. Eng. Sci. Technol. 2017, 52, 526–532. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Yin, K.; Zhu, Z.; Xiao, S.; Wu, Z.; Duan, J.-A. Robust Hierarchical Porous PTFE Film Fabricated via Femtosecond Laser for Self-Cleaning Passive Cooling. Nano Lett. 2021, 21, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Li, Y.; Wang, C.; Zhu, Y.; Wang, H.; Wang, J. Robust Superhydrophobic Epoxy Composite Coating Prepared by Dual Interfacial Enhancement. Chem. Eng. J. 2019, 371, 276–285. [Google Scholar] [CrossRef]
- Xu, P.; Sui, X.; Ge, A.; Wang, S.; Coyle, T.W.; Mostaghimi, J. Hydrocarbon-Induced Reversible Wetting Behaviors of Hierarchically-Structured Yttrium Oxide Coatings. Surf. Coat. Technol. 2022, 449, 128996. [Google Scholar] [CrossRef]
- Ekren, N.; Sarkin, A.S.; Saglam, S. A Hydrophobic Antireflective and Antidust Coating with SiO2 and TiO2 Nanoparticles Using a New 3-D Printing Method for Photovoltaic Panels. IEEE J. Photovolt. 2022, 12, 1014–1026. [Google Scholar] [CrossRef]
- Lv, L.; Liu, H.; Zhang, W.; Chen, J.; Liu, Z. Facile UV-Curable Fabrication of Robust, Anti-Icing Superhydrophobic Coatings Based on Polyurethane. Mater. Lett. 2020, 258, 126653. [Google Scholar] [CrossRef]
- Du, D.; Wang, F.; Zhang, D.; Bao, J.; Fan, Y.; Guo, Y.; Shen, W.; Wang, H. One-Step Synthesis of SiO2 Nanomesh for Antireflection and Self-Cleaning of Solar Cell. J. Colloid Interf. Sci. 2023, 630, 795–803. [Google Scholar] [CrossRef]
- Popescu, V.; Sandu, I.; Muresan, E.I.; Istrate, B.; Lisa, G. Effects of the Pre-Treatment with Atmospheric-Air Plasma Followed by Conventional Finishing. Rev. Chim-Buchar. 2014, 65, 676–683. [Google Scholar] [CrossRef]
- Oh, S.; Cho, J.; Lee, J.; Han, J.; Kim, S.; Nam, Y. A Scalable Haze-Free Antireflective Hierarchical Surface with Self-Cleaning Capability. Adv. Sci. 2022, 9, 2202781. [Google Scholar] [CrossRef]
- Regmi, G.; Velumani, S. Radio frequency (RF) sputtered ZrO2-ZnO-TiO2 coating: An example of multifunctional benefits for thin film solar cells on the flexible substrate. Sol. Energy 2023, 249, 301–311. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Bretler, S.; Cohen, S.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Margel, S. Engineering of Superhydrophobic Silica Microparticles and Thin Coatings on Polymeric Films by Ultrasound Irradiation. Mater. Today Chem. 2021, 21, 100520. [Google Scholar] [CrossRef]
- Music, S.; Filipovic-Vincekovic, N.; Sekovanic, L. Precipitation of Amorphous SiO2 Particles and Their Properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Kuan, H.C.; Chuang, W.P.; Ma, C.C.M. Synthesis and Characterization of a Clay/Waterborne Polyurethane Nanocomposite. J. Mater. Sci. 2005, 40, 179–185. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.; Wu, Z.; Wang, J.; Shen, C.; Liu, Y.; Ren, L. Non-Wet Kingfisher Flying in the Rain: The Water-Repellent Mechanism of Elastic Feathers. J. Colloid Interf. Sci. 2019, 541, 56–64. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent Progress in Antireflection and Self-Cleaning Technology–From Surface Engineering to Functional Surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Z.; Wang, Z.; Wang, Z.; Han, Z. Multifunctional Biomimetic Composite Coating with Antireflection, Self-Cleaning and Mechanical Stability. Nanomaterials 2023, 13, 1855. https://doi.org/10.3390/nano13121855
Jiao Z, Wang Z, Wang Z, Han Z. Multifunctional Biomimetic Composite Coating with Antireflection, Self-Cleaning and Mechanical Stability. Nanomaterials. 2023; 13(12):1855. https://doi.org/10.3390/nano13121855
Chicago/Turabian StyleJiao, Zhibin, Ze Wang, Zhaozhi Wang, and Zhiwu Han. 2023. "Multifunctional Biomimetic Composite Coating with Antireflection, Self-Cleaning and Mechanical Stability" Nanomaterials 13, no. 12: 1855. https://doi.org/10.3390/nano13121855
APA StyleJiao, Z., Wang, Z., Wang, Z., & Han, Z. (2023). Multifunctional Biomimetic Composite Coating with Antireflection, Self-Cleaning and Mechanical Stability. Nanomaterials, 13(12), 1855. https://doi.org/10.3390/nano13121855