Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking
Abstract
:1. Introduction
2. Materials and Methods
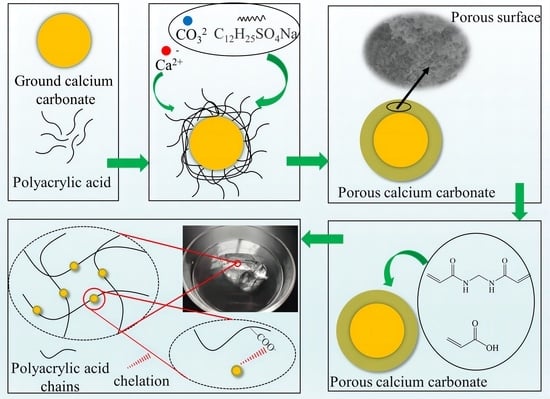
2.1. Preparation of Porous Calcium Carbonate and PCC/PAA
2.2. Characterization Method
2.3. Measurement of Water Absorbency
3. Results and Discussion
3.1. Characterization of PCC/PAA
3.2. Crosslinking Mechanism between Porous Calcium Carbonate and Polymeric Chains
3.3. Property Testing
3.3.1. Water Absorption Test
| Raw Materials | The Ratio of Monomers to the Inorganic Filler | Water Absorbency in Deionized Water (g/g) | Water Absorbency in 0.9 wt% NaCl (g/g) | Ref. |
|---|---|---|---|---|
| Acrylic acid, kaolin | Approximately 13:1 | 670 | N/A | [56] |
| Acrylic acid, oil shale semicoke | Approximately 10: 1 | 420 | 65 | [57] |
| Acrylic acid, bentonite clay | 10:1 | 130 | Approximately 20 | [58] |
| Acrylic acid, montmorillonite | Approximately 7:1 | 400 | N/A | [59] |
| Acrylic acid, acrylamide, montmorillonite, | Approximately 533:1 | 714 | 62 | [60] |
| Acrylamide, montmorillonite | Approximately 15:1 | 721 | Approximately 50 | [61] |
| Acrylic acid, acrylamide, halloysite nanotube | 20:1 | 537 | N/A | [62] |
| PCC (2 wt%)/PAA | 50:1 | 935 | 80 | This work |
3.3.2. Water Absorbency Test in Different pH Solutions and Different Salt Solutions
3.3.3. Water-Retention-Rate Test
3.3.4. Reswelling Water Absorbency Test
3.3.5. Compressive Test
3.4. Mechanism of PCC/PAA Enhanced by Porous Calcium Carbonate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.; Liu, X.; Zhen, J.; Lei, Z. Preparation of superabsorbent resin with fast water absorption rate based on hydroxymethyl cellulose sodium and its application. Carbohydr. Polym. 2019, 225, 115214. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Cui, S.; Wang, X.; Liu, B.; Li, X.; Sun, K.; Peng, H.; Ma, G. Preparation and properties of multifunctional polyaspartic acid/waste paper fiber-based superabsorbent composites. J. Environ. Chem. Eng. 2022, 10, 108405. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Deng, Y.; He, X.; Yang, X.; Chen, R.; Lei, Z. Preparation of superabsorbent polymer gel based on PVPP and its application in water-holding in sandy soil. J. Environ. Chem. Eng. 2021, 9, 106760. [Google Scholar] [CrossRef]
- Zhong, M.; Shi, F.-K.; Liu, Y.-T.; Liu, X.-Y.; Xie, X.-M. Tough superabsorbent poly(acrylic acid) nanocomposite physical hydrogels fabricated by a dually cross-linked single network strategy. Chin. Chem. Lett. 2016, 27, 312–316. [Google Scholar] [CrossRef]
- Choi, H.; Park, J.; Lee, J. Sustainable Bio-Based Superabsorbent Polymer: Poly(itaconic acid) with Superior Swelling Properties. ACS Appl. Polym. Mater. 2022, 4, 4098–4108. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Sang, W.; Liu, B.; Peng, H.; Zhang, W.; Ma, G. Preparation and anti-leakage performances of superabsorbent composite based on ablmoschus manihot gum and microcrystalline cellulose. J. Environ. Chem. Eng. 2022, 10, 107644. [Google Scholar] [CrossRef]
- Oyarce, E.; Cantero-López, P.; Yañez, O.; Roa, K.; Boulett, A.; Pizarro, G.D.C.; Zhang, Y.; Xu, C.; Willför, S.; Sánchez, J. Nanocellulose bio-based composites for the removal of methylene blue from water: An experimental and theoretical exploration. J. Mol. Liq. 2022, 357, 119089. [Google Scholar] [CrossRef]
- Chindasiriphan, P.; Yokota, H.; Kawabata, Y.; Pimpakan, P. Combined effect of rice husk ash and superabsorbent polymer on self-healing capability of mortar. Constr. Build. Mater. 2022, 338, 127588. [Google Scholar] [CrossRef]
- Peng, M.C.; Sethu, V.; Selvarajoo, A. Performance study of chia seeds, chia flour and Mimosa pudica hydrogel as polysaccharide-based superabsorbent polymers for sanitary napkins. Mater. Today Commun. 2021, 26, 101712. [Google Scholar] [CrossRef]
- Chaudhuri, S.D.; Dey, A.; Upganlawar, S.; Chakrabarty, D. Influence of clay concentration on the absorption and rheological attributes of modified cellulose/acrylic acid based hydrogel and the application of such hydrogel. Mater. Chem. Phys. 2022, 282, 125942. [Google Scholar] [CrossRef]
- Hu, X.; Liang, R.; Li, J.; Liu, Z.; Sun, G. Mechanically strong hydrogels achieved by designing homogeneous network structure. Mater. Des. 2019, 163, 107547. [Google Scholar] [CrossRef]
- Ye, Z.; Tang, M.; Hong, X.; Hui, K.S. Sustainable composite super absorbents made from polysaccharides. Mater. Lett. 2016, 183, 394–396. [Google Scholar] [CrossRef]
- Kordjazi, S.; Kamyab, K.; Hemmatinejad, N. Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: An efficient water/oil separation filter. Adv. Compos. Hybrid Mater. 2020, 3, 167–176. [Google Scholar] [CrossRef]
- Kazeminejadfard, F.; Hojjati, M.R. Preparation of superabsorbent composite based on acrylic acid-hydroxypropyl distarch phosphate and clinoptilolite for agricultural applications. J. Appl. Polym. Sci. 2019, 136, 47365. [Google Scholar] [CrossRef]
- Doan, L.; Lu, Y.; Karatela, M.; Phan, V.; Jeffryes, C.; Benson, T.; Wujcik, E.K. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polylactic Acid-Polyethylene Glycol Diblock Copolymer and Graphene Oxide for a Protein Delivery Vehicle. Eng. Sci. 2019, 7, 10–16. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.; Zheng, K.; Ma, Y.; Ding, D.; Zhang, H. Achieving Better Greenhouse Effect than Glass: Visibly Transparent and Low Emissivity Metal-Polymer Hybrid Metamaterials. ES Energy Environ. 2019, 5, 102–107. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, C.; Kim, Y.; Jung, S. Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur. Polym. J. 2020, 127, 109586. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Seggiani, M.; Cinelli, P.; Elnaby, H.M.H.; Azaam, M.M. Swelling capacity of sugarcane bagasse-g-poly(acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020, 133, 109769. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Azaam, M.M.; El-nshar, E.M. Sodium alginate-g-poly(acrylic acid-co-2-hydroxyethyl methacrylate)/montmorillonite superabsorbent composite: Preparation, swelling investigation and its application as a slow-release fertilizer. Arab. J. Chem. 2019, 12, 847–856. [Google Scholar] [CrossRef]
- Fu, E.; Zhang, S.; Luan, Y.; Zhang, Y.; Saghir, S.; Xiao, Z. Novel superabsorbent polymer composites based on α-cellulose and modified zeolite: Synthesis, characterization, water absorbency and water retention capacity. Cellulose 2022, 29, 1727–1737. [Google Scholar] [CrossRef]
- Xiong, H.; Peng, H.; Ye, X.e.; Kong, Y.; Wang, N.; Yang, F.; Meni, B.-H.; Lei, Z. High salt tolerance hydrogel prepared of hydroxyethyl starch and its ability to increase soil water holding capacity and decrease water evaporation. Soil Tillage Res. 2022, 222, 105427. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, S.; Zhen, J.; Lei, Z. Superabsorbent Polymer with Excellent Water/Salt Absorbency and Water Retention, and Fast Swelling Properties for Preventing Soil Water Evaporation. J. Polym. Environ. 2023, 31, 812–824. [Google Scholar] [CrossRef]
- Ritonga, A.H.; Jamarun, N.; Arief, S.; Aziz, H.; Tanjung, D.A.; Isfa, B.; Sisca, V.; Faisal, H. Organic modification of precipitated calcium carbonate nanoparticles as filler in LLDPE/CNR blends with the presence of coupling agents: Impact strength, thermal, and morphology. J. Mater. Res. Technol. 2022, 17, 2326–2332. [Google Scholar] [CrossRef]
- Xie, X.; Ma, L.; Chen, Y.; Luo, X.; Long, M.; Ji, H.; Chen, J. Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy. Nanomaterials 2022, 12, 1459. [Google Scholar] [CrossRef]
- Huang, F.; Liang, Y.; He, Y. On the Pickering emulsions stabilized by calcium carbonate particles with various morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123722. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A Bioinspired Mineral Hydrogel as a Self-Healable, Mechanically Adaptable Ionic Skin for Highly Sensitive Pressure Sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, X. Water absorbency of super absorbent polymer based on flexible polymeric network. Eur. Polym. J. 2022, 166, 111045. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, S.; Chen, Z.; Wei, S.; Sun, Z.; Li, C. Study on surface modification of ground calcium carbonate with novel modifier and its PVC filling performance. Powder Technol. 2022, 412, 118028. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, F.; Huang, Y.; Yang, Q.; Tian, C.; Zhou, W. Preparation of peanut shell-like calcium carbonate from biowaste chicken eggshell and its application for aqueous Victoria Blue B removal. Microporous Mesoporous Mater. 2022, 329, 111549. [Google Scholar] [CrossRef]
- Fiorito, E.; Porcedda, G.E.; Brundu, L.; Passiu, C.; Atzei, D.; Ennas, G.; Elsener, B.; Fantauzzi, M.; Rossi, A. Calcium carbonate as sorbent for lead removal from wastewaters. Chemosphere 2022, 296, 133897. [Google Scholar] [CrossRef]
- Abakumova, T.O.; Gusliakova, O.I.; Cvjetinovic, J.; Efimova, O.I.; Konovalova, E.V.; Schulga, A.A.; Zatsepin, T.S.; Gorin, D.A.; Yashchenok, A.M.; Deyev, S.M. Barnase-Loaded Vaterite Nanoparticles Functionalized by EpCAM Targeting Vectors for the Treatment of Lung Diseases. ACS Appl. Nano Mater. 2022, 5, 10744–10754. [Google Scholar] [CrossRef]
- Tanaka, K.; Tsuchiya, A.; Ogino, Y.; Ayukawa, Y.; Ishikawa, K. Comparison of calcite and vaterite as precursors for CO3Ap artificial bone fabrication through a dissolution–precipitation reaction. Ceram. Int. 2022, 48, 26425–26431. [Google Scholar] [CrossRef]
- Fang, Q.; Song, B.; Tee, T.-T.; Sin, L.T.; Hui, D.; Bee, S.-T. Investigation of dynamic characteristics of nano-size calcium carbonate added in natural rubber vulcanizate. Compos. Part B Eng. 2014, 60, 561–567. [Google Scholar] [CrossRef]
- Shui, M. Polymer surface modification and characterization of particulate calcium carbonate fillers. Appl. Surf. Sci. 2003, 220, 359–366. [Google Scholar] [CrossRef]
- Barhoum, A.; Rahier, H.; Abou-Zaied, R.E.; Rehan, M.; Dufour, T.; Hill, G.; Dufresne, A. Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl. Mater. Interfaces 2014, 6, 2734–2744. [Google Scholar] [CrossRef]
- Mohammadbagheri, Z.; Rahmati, A.; Hoshyarmanesh, P. Synthesis of a novel superabsorbent with slow-release urea fertilizer using modified cellulose as a grafting agent and flexible copolymer. Int. J. Biol. Macromol. 2021, 182, 1893–1905. [Google Scholar] [CrossRef]
- Kaur, S.; Jindal, R. Synthesis of interpenetrating network hydrogel from (gum copal alcohols-collagen)-co-poly(acrylamide) and acrylic acid: Isotherms and kinetics study for removal of methylene blue dye from aqueous solution. Mater. Chem. Phys. 2018, 220, 75–86. [Google Scholar] [CrossRef]
- Pan, H.; Xu, Z.; Wei, Z.; Liu, X.; Xu, M.; Zong, C.; Li, W.; Cui, G.; Cao, L.; Wang, Q. Synergistic Double Cross-Linked Dynamic Network of Epoxidized Natural Rubber/Glycinamide Modified Polyacrylic Acid for Silicon Anode in Lithium Ion Battery: High Peel Strength and Super Cycle Stability. ACS Appl. Mater. Interfaces 2022, 14, 33315–33327. [Google Scholar] [CrossRef]
- Fu, L.-H.; Cao, T.-H.; Lei, Z.-W.; Chen, H.; Shi, Y.-G.; Xu, C. Superabsorbent nanocomposite based on methyl acrylic acid-modified bentonite and sodium polyacrylate: Fabrication, structure and water uptake. Mater. Des. 2016, 94, 322–329. [Google Scholar] [CrossRef]
- Ghaedamini, H.; Amiri, M.C. Effects of temperature and surfactant concentration on the structure and morphology of calcium carbonate nanoparticles synthesized in a colloidal gas aphrons system. J. Mol. Liq. 2019, 282, 213–220. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125133. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, G.; Singh, V.; Rattan, S. A dual stimuli responsive natural polymer based superabsorbent hydrogel engineered through a novel cross-linker. Polym. Chem. 2021, 12, 2404–2420. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiation of Calcium Carbonate Polymorphs by Surface Analysis Techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Miao, S. Investigation on NIR, coating mechanism of PS-b-PAA coated calcium carbonate particulate. Appl. Surf. Sci. 2003, 220, 298–303. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z. Synthesis and characterization of poly(HEA/MALA) hydrogel and its application in removal of heavy metal ions from water. Chem. Eng. J. 2013, 215–216, 894–902. [Google Scholar] [CrossRef]
- Wu, W.; Lu, S.-C. Mechano-chemical surface modification of calcium carbonate particles by polymer grafting. Powder Technol. 2003, 137, 41–48. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, W.; Sun, J. Structural Modifications of Sodium Polyacrylate-Polyacrylamide to Enhance Its Water Absorption Rate. Coatings 2022, 12, 1234. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, A.; Xie, P.; Gao, G.; Xie, L.; Li, X.; Song, A. Synthesis and swelling behaviors of carboxymethyl cellulose-based superabsorbent resin hybridized with graphene oxide. Carbohydr. Polym. 2017, 157, 48–56. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Z.; Meng, H.; Zhang, X. Study on the enhanced adsorption properties of lysozyme on polyacrylic acid modified TiO2 nano-adsorbents. Mater. Res. Express 2018, 5, 015402. [Google Scholar] [CrossRef]
- Li, L.; Jiang, K.; Qian, Y.; Han, H.; Qiao, P.; Zhang, H. Effect of organically intercalation modified layered double hydroxides-graphene oxide hybrids on flame retardancy of thermoplastic polyurethane nanocomposites. J. Therm. Anal. Calorim. 2020, 142, 723–733. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Liu, J.; Mei, H.; Yong, D.; Li, J. Preparation, swelling behaviors and fertilizer-release properties of sodium humate modified superabsorbent resin. Mater. Today Commun. 2019, 19, 124–130. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, L.; Liu, Q.; Yang, M.; Chen, J.; Lei, Z. Preparation and properties of a biodegradability superabsorbent composite based on flax cake protein-g-poly (acrylic acid)/Kaolinite. J. Appl. Polym. Sci. 2021, 139, 51975. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Zhang, C.; Cui, B.; Li, Z.; Zhao, D.; Wang, Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers 2021, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Bi, Y.; Zhou, Y.; Qian, K.; Qian, X.; Zeng, X.; Zhu, Q.; Yu, F.; Ruan, S. Synthesis and characterization of a new super absorbent polymer (SAP) via the use of low-grade kaolin through inverse suspension polymerization. Constr. Build. Mater. 2023, 363, 129849. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Mu, B.; Wang, Y.; Quan, Z.; Wang, A. Synthesis, characterization, and swelling behaviors of sodium carboxymethyl cellulose-g-poly(acrylic acid)/semi-coke superabsorbent. Polym. Bull. 2021, 79, 935–953. [Google Scholar] [CrossRef]
- Datta Chaudhuri, S.; Mandal, A.; Dey, A.; Chakrabarty, D. Tuning the swelling and rheological attributes of bentonite clay modified starch grafted polyacrylic acid based hydrogel. Appl. Clay Sci. 2020, 185, 105405. [Google Scholar] [CrossRef]
- Etemadi Baloch, F.; Afzali, D.; Fathirad, F. Design of acrylic acid/nanoclay grafted polysaccharide hydrogels as superabsorbent for controlled release of chlorpyrifos. Appl. Clay Sci. 2021, 211, 106194. [Google Scholar] [CrossRef]
- Olad, A.; Aghayan, J.; Gharekhani, H.; Akbarzadeh, M. Carboxymethyl cellulose-based semi-IPN hydrogel nanocomposite with improved physicochemical and mechanical properties. J. Polym. Res. 2022, 29, 460. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Azaam, M.M.; El-nshar, E.M. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/montmorillonite superabsorbent composite as a slow-release urea fertilizer. Polym. Adv. Technol. 2018, 29, 2072–2079. [Google Scholar] [CrossRef]
- Menceloglu, Y.; Menceloglu, Y.Z.; Seven, S.A. Triblock Superabsorbent Polymer Nanocomposites with Enhanced Water Retention Capacities and Rheological Characteristics. ACS Omega 2022, 7, 20486–20494. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Rong, J.; Xu, J.; Fang, J. The synthesis of hierarchical porous Al2O3/acrylic resin composites as durable, efficient and recyclable absorbents for oil/water separation. Chem. Eng. J. 2017, 309, 522–531. [Google Scholar] [CrossRef]
- Khushbu; Warkar, S.G.; Kumar, A. Synthesis and assessment of carboxymethyl tamarind kernel gum based novel superabsorbent hydrogels for agricultural applications. Polymer 2019, 182, 121823. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel super-absorbent hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123972. [Google Scholar] [CrossRef]
- Hao, Y.; Qu, J.; Tan, L.; Liu, Z.; Wang, Y.; Lin, T.; Yang, H.; Peng, J.; Zhai, M. Synthesis and property of superabsorbent polymer based on cellulose grafted 2-acrylamido-2-methyl-1-propanesulfonic acid. Int. J. Biol. Macromol. 2023, 233, 123643. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Ran, F.; Dong, Z.; Lei, Z. High performance and low cost composite superabsorbent based on polyaspartic acid and palygorskite clay. Appl. Clay Sci. 2015, 118, 21–28. [Google Scholar] [CrossRef]
- Laguecir, A.; Ulrich, S.; Labille, J.; Fatin-Rouge, N.; Stoll, S.; Buffle, J. Size and pH effect on electrical and conformational behavior of poly(acrylic acid): Simulation and experiment. Eur. Polym. J. 2006, 42, 1135–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, Y.; Mu, B.; Wang, A. Research on preparation and properties of a multifunctional superabsorbent based on semicoke and humic acid. Eur. Polym. J. 2021, 159, 110750. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Zhang, C.; García Meza, J.V.; Zhou, K.; Liu, J.; Song, S.; Zhang, M.; Meng, D.; Chen, J.; Xia, L.; Xiheng, H. Superabsorbent polymer used for saline-alkali soil water retention. J. Taiwan Inst. Chem. Eng. 2023, 145, 104830. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Modibane, K.D.; Kang, M.; Hato, M.J. Sequestration of methylene blue dye using sodium alginate poly(acrylic acid)@ZnO hydrogel nanocomposite: Kinetic, Isotherm, and Thermodynamic Investigations. Int. J. Biol. Macromol. 2020, 162, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Wang, A. Preparation and Properties of Chitosan-g-poly(acrylic acid)/Montmorillonite Superabsorbent Nanocomposite via in Situ Intercalative Polymerization. Ind. Eng. Chem. Res. 2007, 46, 2497–2502. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Su, T.; Chen, Y.; Long, M.; Luo, X.; Xie, X.; Qin, Z. Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking. Nanomaterials 2023, 13, 2575. https://doi.org/10.3390/nano13182575
Jiao Y, Su T, Chen Y, Long M, Luo X, Xie X, Qin Z. Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking. Nanomaterials. 2023; 13(18):2575. https://doi.org/10.3390/nano13182575
Chicago/Turabian StyleJiao, Yixin, Tongming Su, Yongmei Chen, Minggui Long, Xuan Luo, Xinling Xie, and Zuzeng Qin. 2023. "Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking" Nanomaterials 13, no. 18: 2575. https://doi.org/10.3390/nano13182575
APA StyleJiao, Y., Su, T., Chen, Y., Long, M., Luo, X., Xie, X., & Qin, Z. (2023). Enhanced Water Absorbency and Water Retention Rate for Superabsorbent Polymer via Porous Calcium Carbonate Crosslinking. Nanomaterials, 13(18), 2575. https://doi.org/10.3390/nano13182575