Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin
Abstract
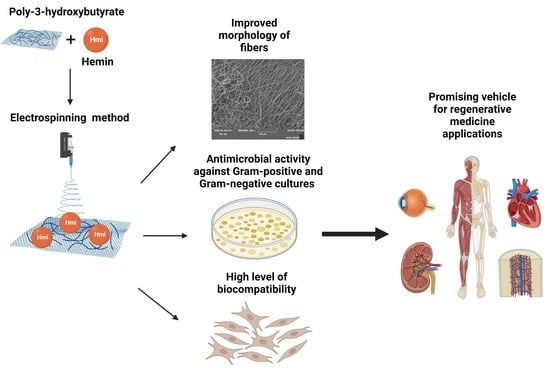
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Electrospun Materials
2.2.2. Scanning Electron Microscopy
2.2.3. Mechanical Analysis
2.2.4. X-ray Diffraction Analysis
2.2.5. Differential Scanning Calorimetry
2.2.6. Wetting Contact Angle
2.2.7. Permeability to Air
2.2.8. Antimicrobial Tests
2.2.9. Hemin Release Studies
2.2.10. Cell Culture
2.2.11. Cytotoxic Activity Analysis
3. Results and Discussion
3.1. Characterization of PHB–Hmi Fibers
3.2. The Barrier Properties of PHB–Hmi Fibers
3.3. The Antimicrobial Tests of PHB–Hmi Fibers
3.4. Cytotoxic Activity Analysis and Biocompatibility of PHB–Hmi Fibers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.K. Biocompatible Polymers and their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef]
- Li, M.; Wilkins, M.R. Recent advances in polyhydroxyalkanoate production: Feedstocks, strains and process developments. Int. J. Biol. Macromol. 2020, 156, 691–703. [Google Scholar] [CrossRef]
- Kushwah, B.S.; Kushwah, A.V.S.; Singh, V. Towards understanding polyhydroxyalkanoates and their use. J. Polym. Res. 2016, 23, 153. [Google Scholar] [CrossRef]
- Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Polyester-based nanoparticles for nucleic acid delivery. Mater. Sci. Eng. C 2018, 92, 983–994. [Google Scholar] [CrossRef]
- Singh, G.; Kumari, A.; Mittal, A.; Yadav, A.; Aggarwal, N.K. Polyβ-Hydroxybutyrate Production by Bacillus subtilisNG220 Using Sugar Industry Waste Water. BioMed Res. Int. 2013, 2013, 952641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, D.; Loh, X.J. Polyhydroxyalkanoates: Chemical Modifications Toward Biomedical Applications. ACS Sustain. Chem. Eng. 2014, 2, 106–119. [Google Scholar] [CrossRef]
- Bakhtiari, S.S.E.; Karbasi, S.; Toloue, E.B. Modified Poly(3-hydroxybutyrate)-based scaffolds in tissue engineering applications: A Review. Int. J. Biol. Macromol. 2021, 166, 986–988. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y. Medical applications of biopolyesters polyhydroxyalkanoates. Chin. J. Polym. Sci. 2013, 31, 719–736. [Google Scholar] [CrossRef]
- Babos, G.; Rydz, J.; Kawalec, M.; Klim, M.; Fodor-Kardos, A.; Trif, L.; Feczkó, T. Poly(3-Hydroxybutyrate)-Based Nanoparticles for Sorafenib and Doxorubicin Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 7312. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Tan, D.; Shang, S.; Wu, X.; Zhao, J.; Ran, G.; Lu, X. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) Biopolyester Based Nanoparticles as NVP-BEZ235 Delivery Vehicle for Tumor Targeting Therapy. Biomacromolecules 2019, 20, 3313–3323. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A hybrid PHB–hydroxyapatite composite for biomedical application: Production, in vitro and in vivo investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Zhang, K.; Liow, S.S.; Loh, X.J. New Dual Functional PHB-Grafted Lignin Copolymer: Synthesis, Mechanical Properties, and Biocompatibility Studies. ACS Appl. Bio Mater. 2018, 2, 127–134. [Google Scholar] [CrossRef]
- Zhijiang, C. Biocompatibility and Biodegradation of novel PHB porous substrates with controlled multi-pore size by emulsion templates method. J. Mater. Sci. Mater. Med. 2006, 17, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Karbasi, S.; Sari Aslani, F.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly (3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. (Shiraz) 2020, 21, 18–30. [Google Scholar] [CrossRef]
- Zhijiang, C.; Qin, Z.; Xianyou, S.; Yuanpei, L. Zein/Poly(3-hydroxybutyrate- co -4-hydroxybutyrate) electrospun blend fiber scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2017, 71, 797–806. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2020, 2020, 111602. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.; Hastings, G.; Оhta, H.; Niwa, S.; Boeree, N. Development of a degradable composite for orthopaedic use: In vivo biomechanical and histological evaluation of two bioactive degradable composites based on the polyhydroxybutyrate polymer. Biomaterials 1992, 13, 491–496. [Google Scholar] [CrossRef]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. Handb. Ecomater. 2017, 1–23. [Google Scholar] [CrossRef]
- Sreedevi, S.; Unni, K.N.; Sajith, S.; Priji, P.; Josh, M.S.; Benjamin, S. Bioplastics: Advances in Polyhydroxybutyrate Research. Adv. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Kumara, B.P.; Maruthi, Y.P.; Pratap, S.; Sudha, K. Development and characterization of polycaprolactone (PCL)/poly ((R)-3-hydroxybutyric acid) (PHB) blend microspheres for tamoxifen drug relese studies. Int. J. Pharm. Pharmac. Sci. 2015, 7, 95–100. [Google Scholar]
- Karimi, A.; Karbasi, S.; Razavi, S.; Zargar, E.N. Poly(hydroxybutyrate)/chitosan Aligned Electrospun Scaffold as a Novel Substrate for Nerve Tissue Engineering. Adv. Biomed. Res. 2018, 7, 44. [Google Scholar] [CrossRef]
- Kim, G.-M.; Wutzler, A.; Radusch, H.-J.; Michler, G.H.; Simon, P.; Sperling, R.A.; Parak, W.J. One-Dimensional Arrangement of Gold Nanoparticles by Electrospinning. Chem. Mater. 2005, 17, 4949–4957. [Google Scholar] [CrossRef]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly(vinyl alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.K.; Bae, J.W.; Park, K.D. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin. Drug Deliv. 2008, 5, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Kumar, K.; Arora, A.; Katti, D.S. Fabrication and characterization of Pluronic modified poly(hydroxybutyrate) fibers for potential wound dressing applications. Mater. Sci. Eng. C 2016, 63, 266–273. [Google Scholar] [CrossRef]
- Seoane, I.; Manfredi, L.; Cyras, V.; Torre, L.; Fortunati, E.; Puglia, D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on Disintegrability in Composting Conditions of Plasticized PHB Nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.; Hansson, F.; Pitkänen, O.; Geng, S.; Oksman, K. Biopolymer Blends of Poly(lactic acid) and Poly(hydroxybutyrate) and Their Functionalization with Glycerol Triacetate and Chitin Nanocrystals for Food Packaging Applications. ACS Appl Polym Mater. 2022, 9, 6592–6601. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Effect of Graphene Oxide on the Properties of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate). Polymers 2021, 13, 2233. [Google Scholar] [CrossRef]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Biotechnol. Appl. Biochem. 2015, 176, 1498–1510. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and antimicrobial activity of biguanidinylated chitosan- g -poly[( R )-3-hydroxybutyrate]. Int. J. Biol. Macromol. 2017, 101, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Wei, H. Integrated nanozymes: Facile preparation and biomedical applications. ChemComm 2018, 54, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.; Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Waghorn, P.A. Radiolabelled porphyrins in nuclear medicine. J. Label. Comp. Radiopharm. 2014, 57, 304–309. [Google Scholar] [CrossRef]
- Chen, Z.; Mai, B.; Tan, H.; Chen, X. Nucleic Acid Based Nanocomposites and Their Applications in Biomedicine. Compos. Commun. 2018, 10, 194–204. [Google Scholar] [CrossRef]
- Yu, W.; Zhen, W.; Zhang, Q.; Li, Y.; Luo, H.; He, J.; Liu, Y.-M. Porphyrin-Based Metal-Organic Frameworks compounds as a promising nanomedicine in photodynamic therapy. ChemMedChem 2020, 15, 1766–1775. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2020, 60, 5010–5035. [Google Scholar] [CrossRef] [Green Version]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef]
- Ruthard, C.; Schmidt, M.; Gröhn, F. Porphyrin-Polymer Networks, Worms, and Nanorods: pH-triggerable Hierarchical Self-assembly. Macromol. Rapid Commun. 2011, 32, 706–711. [Google Scholar] [CrossRef]
- Zhao, L.; Qu, R.; Li, A.; Ma, R.; Shi, L. Cooperative self-assembly of porphyrins with polymers possessing bioactive functions. ChemComm 2016, 52, 13543–13555. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Berry, S.M.; Pfister, T.D. Engineering novel metalloproteins: Design of metal-binding sites into native protein scaffolds. Chem. Rev. 2001, 101, 3047–3080. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Li, B. Self-assembly of hemin on carbon nanotube as highly active peroxidase mimetic and its application for biosensing. RSC Adv. 2013, 3, 6044. [Google Scholar] [CrossRef]
- Qu, R.; Shen, L.; Chai, Z.; Jing, C.; Zhang, Y.; An, Y.; Shi, L. Hemin-block copolymer micelle as an artificial peroxidase and its applications in chromogenic detection and biocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 19207–19216. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Pandit, A.; Farràs, P. Recent advances in the design and sensing applications of hemin/coordination polymer-based nanocomposites. Adv. Mater. 2020, 33, 2003883. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ladan, H.; Gozansky, S.; Malik, Z. Characterization of hemin antibacterial action on Staphylococcus aureus. FEMS Microbiol. Lett. 1987, 48, 401–406. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadamd, E.; Yub, C.Н.; Kimball, W.; Annabi, N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munj, H.R.; Nelson, M.T.; Karandikar, P.S.; Lannutti, J.J.; Tomasko, D.L. Biocompatible electrospun polymer blends for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Macagnano, A. Electrospinning of Polystyrene/Polyhydroxybutyrate Nanofibers Doped with Porphyrin and Graphene for Chemiresistor Gas Sensors. Nanomaterials 2019, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Tyubaeva, P.; Varyan, I.; Lobanov, A.; Olkhov, A.; Popov, A. Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers 2021, 13, 4024. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Kurnosov, A.S.; Karpova, S.G.; Iordanskii, A.L. Structure and Properties of Biopolymeric Fibrous Materials Based on Polyhydroxybutyrate–Metalloporphyrin Complexes. Russ. J. Gen. Chem. 2021, 91, 546–553. [Google Scholar] [CrossRef]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Niamsiri, N. Development and characterization of bio-derived polyhydroxyalkanoate nanoparticles as a delivery system for hydrophobic photodynamic therapy agents. J. Mater. Sci. Mater. Med. 2015, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.M.; Varyan, I.A.; Zykova, A.K.; Yarysheva, A.Y.; Ivchenko, P.V.; Olkhov, A.A.; Arzhakova, O.V. Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers 2022, 14, 4878. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.; Varyan, I.; Krivandin, A.; Shatalova, O.; Karpova, S.; Lobanov, A.; Olkhov, A.; Popov, A. The Comparison of Advanced Electrospun Materials Based on Poly(-3-hydroxybutyrate) with Natural and Synthetic Additives. J. Funct. Biomater. 2022, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the preparation of metalloporphyrins. J. Radioanal. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Lubasova, D.; Martinova, L. Controlled Morphology of Porous Polyvinyl Butyral Nanofibers. J. Nanomater. 2011, 2011, 292516. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.M.; Lee, S.J.; Park, W.H. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Krivandin, A.V.; Solov’eva, A.B.; Glagolev, N.N.; Shatalova, O.V.; Kotova, S.L. Structure alterations of perfluorinated sulfocationic membranes under the action of ethylene glycol (SAXS and WAXS studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- Shibryaeva, L.S.; Shatalova, O.V.; Krivandin, A.V.; Tertyshnaya, Y.V.; Solovova, Y.V. Specific structural features of crystalline regions in biodegradable composites of poly-3-hydroxybutyrate with chitosan. Russ. J. Appl. Chem. 2017, 90, 1443–1453. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Koga, N.; Schick, C.V. Handbook of Thermal Analysis and Calorimetry, Applications to Polymers and Plastics; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; London, UK, 2002. [Google Scholar]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Jedliński, Z. Polymer blends of natural poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly(3-hydroxybutyrate). characterization and biodegradation studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Hantel, M.M.; Armstrong, M.J.; Rosa, F.; l’ Abee, R. Characterization of Tortuosity in Polyetherimide Membranes Based on Gurley and Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2016, 164, A334–A339. [Google Scholar] [CrossRef]
- Arzhakova, O.V.; Nazarov, A.I.; Solovei, A.R.; Dolgova, A.A.; Kopnov, A.Y.; Chaplygin, D.K.; Tyubaeva, P.M.; Yarysheva, A.Y. Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects. Membranes 2021, 11, 834. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Domaschke, S.; Zündel, M.; Mazza, E.; Ehret, A.E. A 3D computational model of electrospun networks and its application to inform a reduced modelling approach. Int. J. Solids Struct. 2019, 178, 76–89. [Google Scholar] [CrossRef]
- Hekmati, A.H.; Rashidi, A.; Ghazisaeidi, R.; Drean, J.-Y. Effect of needle length, electrospinning distance, and solution concentration on morphological properties of polyamide-6 electrospun nanowebs. Text. Res. J. 2013, 83, 1452–1466. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Hermosilla, J.; Navia, R.; Seeger, M. Bacterial polyhydroxybutyrate for electrospun fiber production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interface Sci. 2020, 2020, 102315. [Google Scholar] [CrossRef]
- Cobntbekt, J.; Mabchessault, R.H. Physical properties of poly-β-hydroxybutyrate. J. Mol. Biol. 1972, 71, 735–756. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Ricchelli, F.; Gobbo, S.; Moreno, G.; Salet, C.; Brancaleon, L.; Mazzini, A. Photophysical properties of porphyrin planar aggregates in liposomes. Eur. J. Biochem. 1992, 253, 760–765. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Jung, J.; Ma, W.; Li, L.; Ren, X.; Huang, T.-S. PHB/PCL fibrous membranes modified with SiO2@TiO2-based core@shell composite nanoparticles for hydrophobic and antibacterial applications. RSC Adv. 2019, 9, 23071–23080. [Google Scholar] [CrossRef] [Green Version]
- Zozulia, O.; Korendovych, I.V. Semi-rationally designed short peptides self-assemble and bind hemin to promote cyclopropanation. Angew. Chem. Int. Ed. 2020, 59, 8108–8112. [Google Scholar] [CrossRef] [PubMed]
- Dias, Y.J.; Robles, J.R.; Sinha-Ray, S.; Abiade, J.; Pourdeyhimi, B.; Niemczyk-Soczynska, B.; Yarin, A.L. Solution-Blown Poly(hydroxybutyrate) and ε-Poly-l-lysine Submicro- and Microfiber-Based Sustainable Nonwovens with Antimicrobial Activity for Single-Use Applications. ACS Biomat. Sci. Eng. 2021, 7, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Giannetto, A.; Pagano, P.; Gibbs, E.J. Self-assembly of porphyrins on nucleic acids and polypeptides. J. Am. Chem. Soc. 1991, 113, 7799–7800. [Google Scholar] [CrossRef]











| Diameter of Capillary, mm | Voltage, kV | Distance between the Electrodes, mm | Gas Pressure on the Solution, kg(f)/cm2 |
|---|---|---|---|
| 0.1 | 17–20 | 190–200 | 10–14 |
| Content PHB, wt. % | Content of Hmi, wt. % of PHB Mass | Electrical Conductivity, μS/cm | Viscosity, Pa s |
|---|---|---|---|
| 7 | 0 | 10 | 1.0 |
| 7 | 1 | 11 | 1.4 |
| 7 | 3 | 13 | 1.7 |
| 7 | 5 | 14 | 1.9 |
| Sample | Density, g/cm3 (Mean ± SD, n = 10) | Average Diameter, µm (Mean ± SD, n = 100) | Tensile Strength, MPa (Mean ± SD = 0.05, n = 10) | Elongation at Break, % (Mean ± SD = 0.2, n = 10) |
|---|---|---|---|---|
| PHB 0% wt. | 0.30 ± 0.01 | 3.50 ± 0.08 | 1.7 | 3.6 |
| PHB with 1% wt. of Hmi | 0.20 ± 0.02 | 2.06 ± 0.07 | 0.7 | 4.7 |
| PHB with 3% wt. of Hmi | 0.20 ± 0.01 | 1.77 ± 0.04 | 1.9 | 4.7 |
| PHB with 5% wt. Hmi | 0.17 ± 0.01 | 1.77 ± 0.04 | 5.5 | 6.1 |
| Sample | Concentration of Additive, % | First Heating Run | Second Heating Run | ||
|---|---|---|---|---|---|
| Tm, °C | ΔH, J/g | Tm, °C | ΔH, J/g | ||
| PHB | 0 | 175 | 93.1 | 170 | 90.8 |
| PHB–Hmi | 1 | 172 | 81.8 | 168 | 78.7 |
| PHB–Hmi | 3 | 173 | 77.8 | 170 | 75.4 |
| PHB–Hmi | 5 | 174 | 75.3 | 170 | 72.7 |
| Test Culture | Initial Test Culture, CFU/mL | Sample, CFU/mL | Control, CFU/mL |
|---|---|---|---|
| PHB with 1 % wt. Hmi | |||
| S. aureus р 209 | 2.0 × 104 | 4.5 × 103 | 8.6 × 103 |
| E. coli 1257 | 2.0 × 104 | 8.5 × 102 | 9.8 × 103 |
| S. typhimurium | 2.0 × 104 | 7.2 × 103 | 8.1 × 103 |
| PHB with 3 % wt. Hmi | |||
| S. aureus р 209 | 2.1 × 104 | 1.8 × 103 | 8.6 × 103 |
| E. coli 1257 | 2.0 × 104 | <1 × 102 | 9.8 × 103 |
| S. typhimurium | 2.0 × 104 | 2.1 × 103 | 8.1 × 103 |
| PHB with 5 % wt. Hmi | |||
| S. aureus р 209 | 2.0 × 104 | 0.9 × 103 | 8.6 × 103 |
| E. coli 1257 | 2.0 × 104 | <1 × 102 | 9.8 × 103 |
| S. typhimurium | 2.0 × 104 | 2.0 × 103 | 8.1 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyubaeva, P.M.; Varyan, I.A.; Nikolskaya, E.D.; Mollaeva, M.R.; Yabbarov, N.G.; Sokol, M.B.; Chirkina, M.V.; Popov, A.A. Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials 2023, 13, 236. https://doi.org/10.3390/nano13020236
Tyubaeva PM, Varyan IA, Nikolskaya ED, Mollaeva MR, Yabbarov NG, Sokol MB, Chirkina MV, Popov AA. Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials. 2023; 13(2):236. https://doi.org/10.3390/nano13020236
Chicago/Turabian StyleTyubaeva, Polina M., Ivetta A. Varyan, Elena D. Nikolskaya, Mariia R. Mollaeva, Nikita G. Yabbarov, Maria B. Sokol, Margarita V. Chirkina, and Anatoly A. Popov. 2023. "Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin" Nanomaterials 13, no. 2: 236. https://doi.org/10.3390/nano13020236
APA StyleTyubaeva, P. M., Varyan, I. A., Nikolskaya, E. D., Mollaeva, M. R., Yabbarov, N. G., Sokol, M. B., Chirkina, M. V., & Popov, A. A. (2023). Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials, 13(2), 236. https://doi.org/10.3390/nano13020236