Preparation, Drug Distribution, and In Vivo Evaluation of the Safety of Protein Corona Liposomes for Liraglutide Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prescription Screening
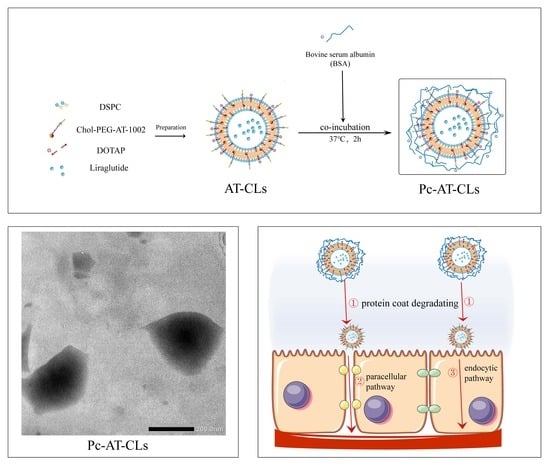
2.3. Preparations of Protein Corona Liposomes
2.4. Characterization of Protein Corona Liposomes
2.4.1. Characterization of Liposome Nanoparticles
2.4.2. Study on Co-Localization
2.4.3. Preparation Characterization by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.5. In Vitro Release and Evaluation of Peptide Stability
2.6. Mucus Co-interaction Measurement
2.7. Transmucosal Transport
2.8. Biosafety Evaluation
2.9. In Vivo Distribution
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Protein Corona Liposomes
3.1.1. Process and Prescription Optimization
3.1.2. Characterization of Liposome Nanoparticles
3.1.3. Co-localization Relationship
3.1.4. Preparation Characterization by SEM and TEM
3.2. In Vitro Release and Evaluation of Peptide Stability
3.3. Mucus Co-Interaction
3.4. Transmucosal Transport
3.5. Biosafety Evaluation
3.6. In Vivo Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonia, T.A.; Sharma, C.P. An Overview of Natural Polymers for Oral Insulin Delivery. Drug Discov. Today 2012, 17, 784–792. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.-J. High-quality Milk Exosomes as Oral Drug Delivery System. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef]
- Agerso, H.; Jensen, L.B.; Elbrond, B.; Rolan, P.; Zdravkovic, M. The Pharmacokinetics; Pharmacodynamics, Safety and Tolerability of NN2211, a New Long-acting GLP-1 Derivative, in Healthy Men. Diabetologia 2002, 45, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Degn, K.B.; Juhl, C.B.; Sturis, J.; Jakobsen, G.; Brock, B.; Chandramouli, V.; Rungby, J.; Landau, B.R.; Schmitz, O. One Week’s Treatment with the Long-acting Glucagon-like Peptide 1 Derivative Liraglutide (NN2211) Markedly Improves 24-h Glycemia and Alpha- and Beta-cell Function and Reduces Endogenous Glucose Release in Patients with Type 2 Diabetes. Diabetes 2004, 53, 1187–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuffer, W.A.; Trujillo, J.M. Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy 2015, 35, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Maji, I.; Mahajan, S.; Sriram, A.; Medtiya, P.; Vasave, R.; Khatri, D.K.; Kumar, R.; Singh, S.B.; Madan, J.; Singh, P.K. Solid Self Emulsifying Drug Delivery System: Superior Mode for Oral Delivery of Hydrophobic Cargos. J. Control. Release 2021, 337, 646–660. [Google Scholar] [CrossRef]
- Pouton, C.W. Lipid Formulations for Oral Administration of Drugs: Non-emulsifying, Self-Emulsifying and ‘Self-microemulsifying’ Drug Delivery Systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld-Van, B.; Verne, G.N. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target Specific Tight Junction Modulators. Adv. Drug Delivery Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Fan, W.; Xia, D.; Zhu, Q.; Hu, L.; Gan, Y. Intracellular Transport of Nanocarriers Across the Intestinal Epithelium. Drug Discov. Today 2016, 21, 856–863. [Google Scholar] [CrossRef]
- Chen, N.; He, Y.; Zang, M.; Zhang, Y.; Lu, H.; Zhao, Q.; Wang, S.; Gao, Y. Approaches and materials for endocytosis-independent intracellular delivery of proteins. Biomaterials 2022, 286, 121567. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, E.; Shi, Y.; Song, B.; Du, H.; Cao, Z. Biomaterial-tight Junction Interaction and Potential Impacts. J. Mater. Chem. B 2019, 7, 6310–6320. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harbor Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tachibana, K.; Krug, S.M.; Kunisawa, J.; Fromm, M.; Kondoh, M. Potential for Tight Junction Protein-directed Drug Development Using Claudin Binders and Angubindin-1. Int. J. Mol. Sci. 2019, 20, 4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielsen, E.M.; Hansen, G.H. Probing Paracellular -versus Transcellular Tissue Barrier Permeability Using a Gut Mucosal Explant Culture System. Tissue Barriers 2019, 7, 1601955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yuan, L.; Zhang, X.; Zhang, A.; Pan, Y.; Wang, Y.; Qu, W.; Hao, H.; Algharib, S.A.; Chen, D.; et al. Core-shell nanosystems designed for effective oral delivery of polypeptide drugs. J. Control. Release 2022, 352, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Fein, K.C.; Gleeson, J.P.; Newby, A.N.; Xian, S.; Cochran, K.; Chaudhary, N.; Melamed, J.R.; Ball, R.L.; Suri, K.; et al. The strawberry-derived permeation enhancer pelargonidin enables oral protein delivery. Proc. Natl. Acad. Sci. USA 2022, 119, e2207829119. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Pandey, N.; Tamiz, A.P.; Vere, J.; Carrasco, R.; Somerville, R.; Tripathi, A.; Ginski, M.; Paterson, B.M.; Alkan, S.S. Mechanism of Action of ZOT-derived Peptide AT-1002, a Tight Junction Regulator and Absorption Enhancer. Int. J. Pharmacol. 2009, 365, 121–130. [Google Scholar] [CrossRef]
- Motlekar, N.A.; Fasano, A.; Wachtel, M.S.; Youan, B.B. Zonula Occludens Toxin Synthetic Peptide Derivative AT1002 Enhances in Vitro and in Vivo Intestinal Absorption of Low Molecular Weight Heparin. J. Drug Target. 2006, 14, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Song, K.-H.; Fasano, A.; Eddington, N.D. Enhanced Nasal Absorption of Hydrophilic Markers after Dosing with AT1002, a Tight Junction Modulator. Eur. J. Pharm. Biopharm. 2008, 69, 231–237. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Durai, M.; Kitchens, K.; Tamiz, A.P.; Somerville, R.; Ginski, M.; Paterson, B.M.; Murray, J.A.; Verdu, E.F.; Alkan, S.S.; et al. Larazotide Acetate Regulates Epithelial Tight Junctions in Vitro and in Vivo. Peptides 2012, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yoosuf, S.; Makharia, G.K. Evolving Therapy for Celiac Disease. Front. Pediatr. 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Liu, L.; Yin, M.; Zhao, Z.; Liang, Y.; Sun, K.; Li, Y. Mucus- and pH-mediated controlled release of core-shell chitosan nanoparticles in the gastrointestinal tract for diabetes treatment. J. Drug Target. 2023, 31, 65–73. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahuja, A.; Baboota, S.; Bhavna; Bali, V.; Saigal, N.; Ali, J. Recent Advances in Pelletization Technique for Oral Drug Delivery: A Review. Curr. Drug Deliv. 2009, 6, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Awasthi, V. Surface Engineering of Liposomes for Stealth Behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.Q.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, J.; Peng, X.L. Synthesis, Characterization and Drug Loading Capacity of Dodecyl Serum Albumin for Insoluble Antitumor Drugs. J. China Pharm. Univ. 2011, 42, 319–323. [Google Scholar]
- Wang, A.; Yang, T.; Fan, W.; Yang, Y.; Zhu, Q.; Guo, S.; Zhu, C.; Yuan, Y.; Zhang, T.; Gan, Y. Protein Corona Liposomes Achieve Efficient Oral Insulin Delivery by Overcoming Mucus and Epithelial Barriers. Adv. Healthcare Mater. 2019, 8, e1801123. [Google Scholar] [CrossRef]
- Passero, F.C., Jr.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The Safety and Efficacy of Onivyde (Irinotecan Liposome Injection) for the Treatment of Metastatic Pancreatic Cancer Following Gemcitabine-based Therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Christin, E.P.; Dittmer, D.P. Liposomal Daunorubicin as Treatment for Kaposi’s Sarcoma. Int. J. Nanomed. 2007, 2, 277–288. [Google Scholar]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, Z.; Li, W.; Guo, L.; Wang, L.; Guo, L.; Ma, S.; Han, B.; Chang, J. pH-sensitive O-carboxymethyl chitosan/sodium alginate nanohydrogel for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2022, 223, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.G.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.Q.; Yang, J.C.; Lamb, N.W.; Cai, S.T.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (peg) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef]
- Akkus, Z.B.; Nazir, I.; Jalil, A.; Tribus, M.; Bernkop-Schnurch, A. Zeta Potential Changing Polyphosphate Nanoparticles: A Promising Approach to Overcome the Mucus and Epithelial Barrier. Mol. Pharmacol. 2019, 16, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, Y.H.; Juang, J.H.; Wey, S.P.; Chen, C.T.; Sung, H.W. In Vivo Evaluation of Safety and Efficacy of Self-Assembled Nanoparticles for Oral Insulin Delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J. Intraluminal pH of the Human Gastrointestinal Tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar] [PubMed]
- Sung, H.W.; Sonaje, K.; Liao, Z.-X.; Hsu, L.W.; Chuang, E.Y. pH-Responsive Nanoparticles Shelled with Chitosan for Oral Delivery of Insulin: From Mechanism to Therapeutic Applications. Acc. Chem. Res. 2011, 45, 619–629. [Google Scholar] [CrossRef]
- Takatsuka, S.; Kitazawa, T.; Morita, T.; Horikiri, Y.; Yoshino, H. Enhancement of Intestinal Absorption of Poorly Absorbed Hydrophilic Compounds by Simultaneous Use of Mucolytic Agent and Non-Ionic Surfactant. Eur. J. Pharm. Biopharm. 2006, 62, 52–58. [Google Scholar] [CrossRef]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the Fate of Polymeric Nanoparticles in the Process of the Intestinal Absorption Based on Model Nanoparticles with Various Characteristics: Size, Surface Charge and Pro-hydrophobics. J. Nanobiotechnol. 2021, 19, 32. [Google Scholar] [CrossRef]
- Hu, S.; Yang, Z.; Wang, S.; Wang, L.; He, Q.; Tang, H.; Chen, T. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem. Eng. J. 2022, 428, 132107. [Google Scholar] [CrossRef]








| Investigation Factors | Single Factor Ratio | ||
|---|---|---|---|
| DSPC: Chol (mg/mg) | 2:1 | 3:1 | 4:1 |
| DOTAP: Chol (mg/mg) | 1:1 | 2:1 | 3:1 |
| Drug: Lipid (mg/mg) | 1:5 | 1:10 | 1:15 |
| Concentration of BSA (mg/mL) | 5 | 10 | 15 |
| BSA: CLs (v/v) | 1:1 | 2:1 | 3:1 |
| Internal water phase to oil phase (v/v) | 100:1000 | 200:1000 | 300:1000 |
| Ultrasonic Power of Colostrum (W) | Ultrasonic Power of Double Milk (W) | Zeta Potential (mV) | Size (nm) | Polydisperse Index (PDI) | Encapsulation Efficiency (EE%) |
|---|---|---|---|---|---|
| 150 | 150 | 24.78 | 144.9 | 0.26 | 73% |
| 80 | 150 | 21.54 | 140 | 0.24 | 54% |
| 80 | 200 | 21.44 | 122.7 | 0.24 | 78% |
| 80 | 250 | 27.51 | 120 | 0.29 | 87% |
| Formations | Size (nm) | Zeta (mV) | Encapsulation Efficiency (EE%) | Drug Loading (DL%) |
|---|---|---|---|---|
| CLs | 127 ± 10.0 | 36.09 ± 2.7 | 85.85 ± 1.79 | 8.19 ± 0.17 |
| AT-CLs | 119.6 ± 5.6 | 36.14 ± 0.16 | 84.63 ± 5.05 | 8.08 ± 0.48 |
| PcCLs | 209.0 ± 9.6 | 0.67 ± 0.36 | 85.85 ± 1.79 | 2.92 ± 1.67 |
| Pc-AT-CLs | 202.9 ± 12.4 | 1.76 ± 4.87 | 84.63 ± 5.05 | 2.08 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Zhao, Z.; He, J.; Tao, Y.; Zhang, H.; Yuan, R.; Sun, K.; Shi, Y. Preparation, Drug Distribution, and In Vivo Evaluation of the Safety of Protein Corona Liposomes for Liraglutide Delivery. Nanomaterials 2023, 13, 540. https://doi.org/10.3390/nano13030540
Ding R, Zhao Z, He J, Tao Y, Zhang H, Yuan R, Sun K, Shi Y. Preparation, Drug Distribution, and In Vivo Evaluation of the Safety of Protein Corona Liposomes for Liraglutide Delivery. Nanomaterials. 2023; 13(3):540. https://doi.org/10.3390/nano13030540
Chicago/Turabian StyleDing, Ruihuan, Zhenyu Zhao, Jibiao He, Yuping Tao, Houqian Zhang, Ranran Yuan, Kaoxiang Sun, and Yanan Shi. 2023. "Preparation, Drug Distribution, and In Vivo Evaluation of the Safety of Protein Corona Liposomes for Liraglutide Delivery" Nanomaterials 13, no. 3: 540. https://doi.org/10.3390/nano13030540
APA StyleDing, R., Zhao, Z., He, J., Tao, Y., Zhang, H., Yuan, R., Sun, K., & Shi, Y. (2023). Preparation, Drug Distribution, and In Vivo Evaluation of the Safety of Protein Corona Liposomes for Liraglutide Delivery. Nanomaterials, 13(3), 540. https://doi.org/10.3390/nano13030540