Fluorometric Sensing and Detection of p-Nitroaniline by Mixed Metal (Zn, Ni) Tungstate Nanocomposite
Abstract
:1. Introduction
2. Methods and Material
2.1. Chemicals and Reagents
2.2. Synthesis of Nanoparticles
2.2.1. Synthesis of ZnWO4 Nanoparticles
2.2.2. Synthesis of NiWO4 Nanoparticles
2.2.3. Synthesis of ZnNiWO4 Nanocomposite
2.3. Characterization of the Synthesized Materials
2.4. Sensing Experiment
3. Results and Discussion
3.1. Material Characterization
3.2. Photoluminescence Studies for Detection of p-Nitroaniline
3.2.1. Effect of Solvent
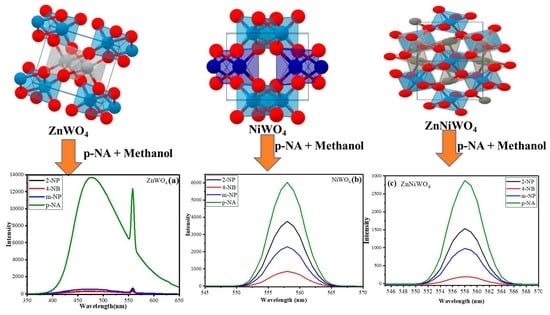
3.2.2. Selectivity of Nitro-Compound
3.2.3. Effect of p-NA Concentration
3.3. Anti-Interference Test
3.4. Recyclability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, G.; Das, P.; Mandal, S.K. Strategic Construction of Highly Stable Metal-Organic Frameworks Combining Both Semi-Rigid Tetrapodal and Rigid Ditopic Linkers: Selective and Ultrafast Sensing of 4-Nitroaniline in Water. ACS Appl. Mater. Interfaces 2018, 10, 42406–42416. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Wang, N.; Li, Y.; Fu, D.; Fan, H.; Luo, M.; Yue, S. A New Three-Dimensional Cd(II) Metal-Organic Framework for Highly Selective Sensing of Fe3+as Well as Nitroaromatic Compounds. ChemistrySelect 2017, 2, 12046–12050. [Google Scholar] [CrossRef]
- Vishnoi, P.; Walawalkar, M.G.; Sen, S.; Datta, A.; Patwari, G.N.; Murugavel, R. Selective Fluorescence Sensing of Polynitroaromatic Explosives Using Triaminophenylbenzene Scaffolds. Phys. Chem. Chem. Phys. 2014, 16, 10651–10658. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Campos, B.B.; Miranda, M.S.; da Silva, J.C.G.E. CdSe Quantum Dots Capped PAMAM Dendrimer Nanocomposites for Sensing Nitroaromatic Compounds. Talanta 2011, 83, 1335–1340. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, Y.; Su, X.; Ma, Q. Nitrogen-Rich Quantum Dots-Based Fluorescence Molecularly Imprinted Paper Strip for p-Nitroaniline Detection. Microchem. J. 2019, 148, 162–168. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E.S. Photocatalytic Conversion of 4-Nitroaniline to p-Phenylenediamine Using Ni/ZnSn(OH)6 Nanoparticles. J. Ind. Eng. Chem. 2014, 20, 3329–3334. [Google Scholar] [CrossRef]
- Sajid, H.; Asif, M.; Ayub, K.; Gilani, M.A.; Akhter, M.S.; Mahmood, T. Novel Microporous B6N6 Covalent Organic Framework (COF) as an Electrochemical Sensor for the Ultra-Selective Detection of Nitroaniline Isomers; a DFT Outcome. Surf. Interfaces 2021, 27, 101587. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Mofazzeli, F.; Es’haghi, Z. Determination of 3-Nitroaniline in Water Samples by Directly Suspended Droplet Three-Phase Liquid-Phase Microextraction Using 18-Crown-6 Ether and High-Performance Liquid Chromatography. J. Chromatogr. A 2009, 1216, 5086–5091. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, F.; Lin, X.; Wang, C.; Fu, Y.; Wang, X.; Zhao, Y.; Li, G. Selective Fluorescence Sensors for Detection of Nitroaniline and Metal Ions Based on Ligand-Based Luminescent Metal-Organic Frameworks. J. Solid State Chem. 2015, 232, 96–101. [Google Scholar] [CrossRef]
- Yan, X.; Chen, J.Q.; Hu, M.L.; Sakiyama, H.; Muddassir, M.; Liu, J.Q. Syntheses, Structures and Mechanisms of Interactions with DNA of Two New 20-Core Silver(I) Complexes with Different Ligands. Inorg. Chim. Acta 2023, 546, 121297. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, G.Y.; Liu, X.J.; Feng, R.; Zhang, H.J.; Zhang, S.Y.; Zhang, Y.H. A Fluorescent Anthracene-Based Metal–Organic Framework for Highly Selective Detection of Nitroanilines. Inorg. Chim. Acta 2018, 473, 70–74. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Pongprom, A.; Chansud, N.; Bunkoed, O. A Fluorescence Sensor Probe Based on Porous Carbon, Molecularly Imprinted Polymer and Graphene Quantum Dots for the Detection of Trace Sulfadimethoxine. J. Photochem. Photobiol. A Chem. 2022, 427, 113812. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Wang, L. Nanomaterials for Luminescence Detection of Nitroaromatic Explosives. TrAC Trends Anal. Chem. 2015, 65, 13–21. [Google Scholar] [CrossRef]
- Kushwaha, C.S.; Singh, P.; Shukla, S.K.; Chehimi, M.M. Advances in Conducting Polymer Nanocomposite Based Chemical Sensors: An Overview. Mater. Sci. Eng. B 2022, 284, 115856. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Krishnapandi, A.; Chen, S.M.; Balamurugan, K.; Chang, F.M.; Sakthinathan, S. Electrochemical Investigation of Zinc Tungstate Nanoparticles; a Robust Sensor Platform for the Selective Detection of Furazolidone in Biological Samples. Microchem. J. 2021, 160, 105750. [Google Scholar] [CrossRef]
- Mgidlana, S.; Sen, P.; Nyokong, T. Photodegradation of Tetracycline by Asymmetrical Zinc (II)Phthalocyanines Conjugated to Cobalt Tungstate Nanoparticles. J. Mol. Struct. 2022, 1261, 132938. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Elseman, A.M.; Harraz, F.A.; Ahmed, Y.M.Z.; El-Sheikh, S.M.; Rashad, M.M. Superior UV-Light Photocatalysts of Nano-Crystalline (Ni or Co) FeWO4: Structure, Optical Characterization and Synthesis by a Microemulsion Method. New J. Chem. 2021, 45, 3150–3159. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Y.; Guo, J.; Zhao, L.; Wang, T.; Yan, X.; Wang, C.; Liu, F.; Sun, P.; Lu, G. Highly Sensitive and Selective Xylene Sensor Based on P-p Heterojunctions Composites Derived from off-Stoichiometric Cobalt Tungstate. Sens. Actuators B Chem. 2022, 351, 130973. [Google Scholar] [CrossRef]
- Ghorui, U.K.; Satra, J.; Mondal, P.; Mardanya, S.; Sarkar, A.; Srivastava, D.N.; Adhikary, B.; Mondal, A. Graphitic Carbon Nitride Embedded-Ag Nanoparticle Decorated-ZnWO4 Nanocomposite-Based Photoluminescence Sensing of Hg2+. Mater. Adv. 2021, 2, 4041–4057. [Google Scholar] [CrossRef]
- Veldurthi, N.K.; Eswar, N.K.; Singh, S.A.; Madras, G. Heterojunction ZnWO4/ZnFe2O4 Composites with Concerted Effects and Integrated Properties for Enhanced Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2018, 8, 1083–1093. [Google Scholar] [CrossRef]
- Siriwong, P.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Hydrothermal Synthesis, Characterization, and Optical Properties of Wolframite ZnWO4 Nanorods. CrystEngComm 2011, 13, 1564–1569. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Gonçalves, R.F.; Gracia, L.; Andrés, J.; Longo, E. ZnWO4 Nanocrystals: Synthesis, Morphology, Photoluminescence and Photocatalytic Properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Wang, X.; Cui, R.; Chen, S.; Zhou, Y. A Novel Electrochemical Sensor for the Selective Determination of Hydroquinone and Catechol Using Synergic Effect of Electropolymerized Nicotinic Acid Film and Cd-Doped ZnWO4 Nanoneedle. J. Electroanal. Chem. 2019, 834, 196–205. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, J.; He, Y.X.; Li, B.H.; Liu, J.Q.; Prasad, R.; Kumar, A.; Batten, S.R. A 3D Luminescent Zn(II) MOF for the Detection of High Explosives and the Degradation of Organic Dyes: An Experimental and Computational Study. CrystEngComm 2017, 19, 6464–6472. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Zhu, Q.; Li, J.G.; Kim, B.N. Crystallization and Architecture Engineering of ZnWO4 for Enhanced Photoluminescence. CrystEngComm 2020, 22, 6398–6406. [Google Scholar] [CrossRef]
- Amari, R.; Benrezgua, E.; Deghfel, B.; Abdelhalim, Z.; Kamil Yaakob, M.; Jeffrey Basirun, W.; Boukhari, A.; Kheawhom, S.; Azmin Mohamad, A. Ni Doping Effect on the Electronic, Structural and Optical Properties of ZnO Nanoparticles Prepared by Co-Precipitation Route. Opt. Mater. 2022, 128, 112398. [Google Scholar] [CrossRef]
- Geetha, G.v.; Sivakumar, R.; Sanjeeviraja, C.; Ganesh, V. Photocatalytic Degradation of Methylene Blue Dye Using ZnWO4 Catalyst Prepared by a Simple Co-Precipitation Technique. J. Sol-Gel Sci. Technol. 2021, 97, 572–580. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A Microporous 2D Cobalt-Based MOF with Pyridyl Sites and Open Metal Sites for Selective Adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A New 3D 8-Connected Cd(II) MOF as a Potent Photocatalyst for Oxytetracycline Antibiotic Degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Shanmugapriya, S.; Nithya, V.D.; Rajalakshmi, A.; Sivaranjani, K.S.; Bharathi, P.; Shalini, S.; Kasturi, P.R.; Selvan, R.K. Sonochemical Synthesis, Structural, Electrical Transport and Magnetic Properties of NiWO4 Nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 15616–15626. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alanazi, H.S.; Abdullah Alsyahi, A.; Ahmad Citation, N.; Hydrothermal Synthesis, N.; Syrek, K.; Vergara, A. Hydrothermal Synthesis, Characterization and Exploration of Photocatalytic Activities of Polyoxometalate: Ni-CoWO4 Nanoparticles. Crystals 2021, 11, 456. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung Der Größe Und Der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen Math. Phys. Kl. 1918, 26, 98–100. [Google Scholar]
- Kraus, H.; Mikhailik, V.B.; Ramachers, Y.; Day, D.; Hutton, K.B.; Telfer, J. Feasibility Study of a ZnWO4 Scintillator for Exploiting Materials Signature in Cryogenic WIMP Dark Matter Searches. Phys. Lett. B 2005, 610, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Rui, Y.; Sun, K.; Cui, W.; An, W. Surface Decoration of ZnWO4 Nanorods with Cu2O Nanoparticles to Build Heterostructure with Enhanced Photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.; Zhang, W.; Hu, Z.; Feng, Z.; Hu, P.; Wu, H.; Ma, L.; Pan, Y.; Zhu, Y.; Xiong, G. Dual-Functional Bi3+, Dy3+ Co-Doping ZnWO4 for Photoluminescence and Photocatalysis. Appl. Phys. A 2018, 124, 1–8. [Google Scholar] [CrossRef]
- Daemi, S.; Moalem-Banhangi, M.; Ghasemi, S.; Ashkarran, A.A. An Efficient Platform for the Electrooxidation of Formaldehyde Based on Amorphous NiWO4 Nanoparticles Modified Electrode for Fuel Cells. J. Electroanal. Chem. 2019, 848, 113270. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Zeng, D.; Yu, C.; Zhu, L.; Huang, W.; Yang, K.; Li, D. A Facile Microwave-Hydrothermal Method to Fabricate B Doped ZnWO4 Nanorods with High Crystalline and Highly Efficient Photocatalytic Activity. Mater. Res. Bull. 2017, 94, 298–306. [Google Scholar] [CrossRef]
- Umadevi, M.; Vanelle, P.; Terme, T.; Rajkumar, B.J.M.; Ramakrishnan, V. Spectral Investigations of Solvatochromism and Preferential Solvation on 1,4-Dihydroxy-2,3-Dimethyl-9,10-Anthraquinone. J. Fluoresc. 2008, 18, 1139–1149. [Google Scholar] [CrossRef]
- Ren, G.; Tang, M.; Chai, F.; Wu, H. One-Pot Synthesis of Highly Fluorescent Carbon Dots from Spinach and Multipurpose Applications. Eur. J. Inorg. Chem. 2018, 2018, 153–158. [Google Scholar] [CrossRef]
- Mishra, A.; Dheepika, R.; Parvathy, P.A.; Imran, P.M.; Bhuvanesh, N.S.P.; Nagarajan, S. Fluorescence Quenching Based Detection of Nitroaromatics Using Luminescent Triphenylamine Carboxylic Acids. Sci. Rep. 2021, 11, 19324. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, K.; Balasaravanan, R.; Siva, A. Electron Rich Triphenylamine Derivatives (D-π-D) for Selective Sensing of Picric Acid in Aqueous Media. Sens. Actuators B Chem. 2016, 231, 302–312. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.Y.; You, S.Y. A Luminescent Pillar-Layer Zn(II) Metal–Organic Framework for the Ultrasensitive Detection of Nitroaniline. Inorg. Chim. Acta 2020, 509, 119703. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Hahn, Y.B. Development of Highly-Stable Binder-Free Chemical Sensor Electrodes for p-Nitroaniline Detection. J. Colloid Interface Sci. 2017, 494, 300–306. [Google Scholar] [CrossRef]
- Bakhsh, E.M.; Ali, F.; Khan, S.B.; Marwani, H.M.; Danish, E.Y.; Asiri, A.M. Copper Nanoparticles Embedded Chitosan for Efficient Detection and Reduction of Nitroaniline. Int. J. Biol. Macromol. 2019, 131, 666–675. [Google Scholar] [CrossRef]
- Wei, F.; Cai, X.; Nie, J.; Wang, F.; Lu, C.; Yang, G.; Chen, Z.; Ma, C.; Zhang, Y. A 1,2,3-Triazolyl Based Conjugated Microporous Polymer for Sensitive Detection of p-Nitroaniline and Au Nanoparticle Immobilization. Polym. Chem. 2018, 9, 3832–3839. [Google Scholar] [CrossRef]
- de Barros, M.R.; Winiarski, J.P.; Elias, W.C.; de Campos, C.E.M.; Jost, C.L. Au-on-Pd Bimetallic Nanoparticles Applied to the Voltammetric Determination and Monitoring of 4-Nitroaniline in Environmental Samples. J. Environ. Chem. Eng. 2021, 9, 105821. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, S.; Wu, W.; Peng, H.; Yu, Y.; Zhu, D. Cucurbit[6]Uril Modified CdTe Quantum Dots Fluorescent Probe and Its Selective Analysis of p-Nitroaniline in Environmental Samples. Talanta 2019, 199, 667–673. [Google Scholar] [CrossRef]
- Laghrib, F.; Ajermoun, N.; Bakasse, M.; Lahrich, S.; el Mhammedi, M.A. Synthesis of Silver Nanoparticles Assisted by Chitosan and Its Application to Catalyze the Reduction of 4-Nitroaniline. Int. J. Biol. Macromol. 2019, 135, 752–759. [Google Scholar] [CrossRef]














| Component | 2θ | FWHM (βhkl) | Interlayer Spacing (d111) (A°) | Size of Crystal (nm) at (111) | Dislocation Density (δ) × 1019 Lines (m−2) | % Crystallinity (%) |
|---|---|---|---|---|---|---|
| NiWO4 | 31.02 | 0.52 | 2.88 | 15.81 | 1.21 | 72.83 |
| ZnWO4 | 30.62 | 0.57 | 2.91 | 14.43 | 1.18 | 71.35 |
| ZnNiWO4 | 30.77 | 0.60 | 2.90 | 13.67 | 1.19 | 60.87 |
| Material | KSV | R2 | LOD (M) |
|---|---|---|---|
| ZnWO4 | 0.015 | 0.98 | 1.93 × 10−8 |
| NiWO4 | 0.016 | 0.98 | 2.17 × 10−8 |
| ZnNiWO4 | 0.018 | 0.97 | 2.98 × 10−8 |
| Sensors | Methods | LOD (M) | Reference |
|---|---|---|---|
| Zn (II)-MOF | Fluorescence | 4.7 × 10−5 | [44] |
| ZnO NRs/fluorine-doped tin oxide | Electrochemical | 0.5 × 10−6 | [45] |
| Copper nanoparticles-embedded chitosan | Electrochemical | 0.37 × 10−6 | [46] |
| 1,2,3-triazolyl PTPTB | Fluorescence | 4.2 × 10−6 | [47] |
| Au-on-Pd NP | Voltametric | 0.17 × 10−6 | [48] |
| Cucurbituril-modified CdTe quantum dots (CB@QDs) | Fluorescence | 6 × 10−8 | [49] |
| Chitosan-Ag NPs/CPE | Voltametric | 0.86 × 10−6 | [50] |
| ZnNiWO4 NPs | Fluorescence | 2.98 × 10−8 | Present Study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, F.A.; Aldubeikl, H.K.; Alanazi, H.S.; Al-Nafaei, W.S.; Hasan, I. Fluorometric Sensing and Detection of p-Nitroaniline by Mixed Metal (Zn, Ni) Tungstate Nanocomposite. Nanomaterials 2023, 13, 362. https://doi.org/10.3390/nano13020362
Alharthi FA, Aldubeikl HK, Alanazi HS, Al-Nafaei WS, Hasan I. Fluorometric Sensing and Detection of p-Nitroaniline by Mixed Metal (Zn, Ni) Tungstate Nanocomposite. Nanomaterials. 2023; 13(2):362. https://doi.org/10.3390/nano13020362
Chicago/Turabian StyleAlharthi, Fahad A., Hend Khalid Aldubeikl, Hamdah S. Alanazi, Wedyan Saud Al-Nafaei, and Imran Hasan. 2023. "Fluorometric Sensing and Detection of p-Nitroaniline by Mixed Metal (Zn, Ni) Tungstate Nanocomposite" Nanomaterials 13, no. 2: 362. https://doi.org/10.3390/nano13020362
APA StyleAlharthi, F. A., Aldubeikl, H. K., Alanazi, H. S., Al-Nafaei, W. S., & Hasan, I. (2023). Fluorometric Sensing and Detection of p-Nitroaniline by Mixed Metal (Zn, Ni) Tungstate Nanocomposite. Nanomaterials, 13(2), 362. https://doi.org/10.3390/nano13020362