Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth
Abstract
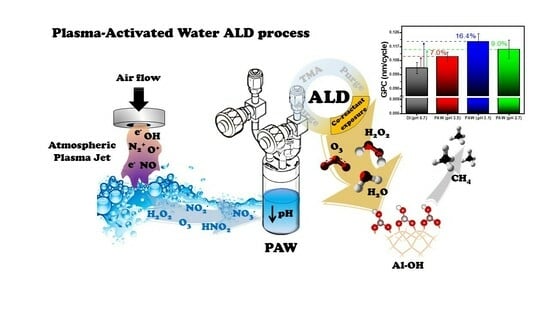
:1. Introduction
2. Materials and Methods
2.1. PAW Synthesis and Characterization
2.2. Al2O3 Thin Film Deposition and In Situ Process Monitoring
2.3. Material Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; Chiappim, W.; Sampaio, A.d.G.; Vegian, M.R.d.C.; Pessoa, R.S.; Koga-Ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; (Ken) Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contributions to the chemistry of plasma-activated water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 2250–2268. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Wu, R.A.; Liao, X.; Liu, D.; Cullen, P.J.; Zhou, R.-W.; Ding, T. Diagnostic analysis of reactive species in plasma-activated water (PAW): Current advances and outlooks. J. Phys. D Appl. Phys. 2022, 55, 023002. [Google Scholar] [CrossRef]
- Galář, P.; Khun, J.; Fučíková, A.; Dohnalová, K.; Popelář, T.; Matulková, I.; Valenta, J.; Scholtz, V.; Kůsová, K. Non-thermal pulsed plasma activated water: Environmentally friendly way for efficient surface modification of semiconductor nanoparticles. Green Chem. 2021, 23, 898–911. [Google Scholar] [CrossRef]
- Sharmin, N.; Pang, C.; Sone, I.; Walsh, J.L.; Fernández, C.G.; Sivertsvik, M.; Fernández, E.N. Synthesis of Sodium Alginate–Silver Nanocomposites Using Plasma Activated Water and Cold Atmospheric Plasma Treatment. Nanomaterials 2021, 11, 2306. [Google Scholar] [CrossRef]
- Sampaio, A.d.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893. [Google Scholar] [CrossRef]
- Chen, L.; Warburton, R.E.; Chen, K.-S.; Libera, J.A.; Johnson, C.; Yang, Z.; Hersam, M.C.; Greeley, J.P.; Elam, J.W. Mechanism for Al2O3 Atomic Layer Deposition on LiMn2O4 from in Situ Measurements and Ab Initio Calculations. Chem 2018, 4, 2418–2435. [Google Scholar] [CrossRef]
- Seo, S.; Nam, T.; Lee, H.-B.-R.; Kim, H.; Shong, B. Molecular oxidation of surface –CH3 during atomic layer deposition of Al2O3 with H2O, H2O2, and O3: A theoretical study. Appl. Surf. Sci. 2018, 457, 376–380. [Google Scholar] [CrossRef]
- Elliott, S.D.; Scarel, G.; Wiemer, C.; Fanciulli, M.; Pavia, G. Ozone-Based Atomic Layer Deposition of Alumina from TMA: Growth, Morphology, and Reaction Mechanism. Chem. Mater. 2006, 18, 3764–3773. [Google Scholar] [CrossRef]
- Nam, T.; Lee, H.; Seo, S.; Cho, S.M.; Shong, B.; Lee, H.-B.-R.; Kim, H. Moisture barrier properties of low-temperature atomic layer deposited Al2O3 using various oxidants. Ceram. Int. 2019, 45, 19105–19112. [Google Scholar] [CrossRef]
- Fan, J.-F.; Sugioka, K.; Toyoda, K. Low-Temperature Growth of Thin Films of Al2O3 by Sequential Surface Chemical Reaction of trimethylaluminum and H2O2. Jpn. J. Appl. Phys. 1991, 30, L1139. [Google Scholar] [CrossRef]
- Yasuhito, K.; Hayato, M.; Hiroshi, T.; Tadaki, M.; Takashi, K.; Nobuyasu, T.; Spiegelman, J. ALD Process Using Hydrogen Peroxide (H2O2mix) for High Aspect Ratio Structures. TAIYO NIPPON SANSO Technical Report No.4. 2022. Available online: https://www.tn-sanso.co.jp/Portals/0/resources/en/rd/giho/pdf/41/tnscgiho41_E03.pdf (accessed on 2 December 2023).
- Heil, S.B.S.; van Hemmen, J.L.; van de Sanden, M.C.M.; Kessels, W.M.M. Reaction mechanisms during plasma-assisted atomic layer deposition of metal oxides: A case study for Al2O3. J. Appl. Phys. 2008, 103, 103302. [Google Scholar] [CrossRef]
- Rose, M.; Niinistö, J.; Endler, I.; Bartha, J.W.; Kücher, P.; Ritala, M. In Situ Reaction Mechanism Studies on Ozone-Based Atomic Layer Deposition of Al2O3 and HfO2. ACS Appl. Mater. Interfaces 2010, 2, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Kayanuma, M.; Choe, Y.-K.; Hagiwara, T.; Kameda, N.; Shimoi, Y. Theoretical Study of the Mechanism for the Reaction of Trimethylaluminum with Ozone. ACS Omega 2021, 6, 26282–26292. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhao, P.; Yi, X.; Shen, X.; Xu, Y. Mechanical Strengths and Thermal Properties of Titania-Doped Alumina Aerogels and the Application as High-Temperature Thermal Insulator. J. Sol-Gel Sci. Technol. 2019, 91, 514–522. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, H.; Chen, Z.; Xiong, P.; Xu, X.; Chen, F.; Li, K.; Duan, Y. Effect of Various Oxidants on Reaction Mechanisms, Self-Limiting Natures and Structural Characteristics of Al2O3 Films Grown by Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 1701248. [Google Scholar] [CrossRef]
- Katamreddy, R.; Inman, R.; Jursich, G.; Soulet, A.; Takoudis, C. ALD and Characterization of Aluminum Oxide Deposited on Si(100) using Tris(diethylamino) Aluminum and Water Vapor. J. Electrochem. Soc. 2006, 153, C701–C706. [Google Scholar] [CrossRef]
- Castillo-Saenz, J.; Nedev, N.; Valdez-Salas, B.; Curiel-Alvarez, M.; Mendivil-Palma, M.I.; Hernandez-Como, N.; Martinez-Puente, M.; Mateos, D.; Perez-Landeros, O.; Martinez-Guerra, E. Properties of Al2O3 Thin Films Grown by PE-ALD at Low Temperature Using H2O and O2 Plasma Oxidants. Coatings 2021, 11, 1266. [Google Scholar] [CrossRef]
- Doria, A.C.O.C.; Figueira, F.R.; De Lima, J.S.B.; Figueira, J.A.N.; Castro, A.H.R.; Sismanoglu, B.N.; Petraconi, G.; Maciel, H.S.; Khouri, S.; Pessoa, R.S. Inactivation of Candida Albicans Biofilms by Atmospheric Gliding Arc Plasma Jet: Effect of Gas Chemistry/Flow and Plasma Pulsing. Plasma Res. Express 2019, 1. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.; da, G.; Miranda, F.; Fraga, M.; Petraconi, G.; da Silva Sobrinho, A.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus Aureus, Escherichia Coli, and Candida Albicans. Water 2021, 13, 1480. [Google Scholar] [CrossRef]
- Oh, J.S.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV-Vis Spectroscopy Study of Plasma-Activated Water: Dependence of the Chemical Composition on Plasma Exposure Time and Treatment Distance. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, D.; He, T.; Guo, L.; Xu, D.; Kong, M.G. Quantifying the Concentration and Penetration Depth of Long-Lived RONS in Plasma-Activated Water by UV Absorption Spectroscopy. AIP Adv. 2019, 9. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Examination of UV-Absorption Spectroscopy for Analysis of O3, NO2−, and HNO2 Compositions and Kinetics in Plasma-Activated Water. Jpn. J. Appl. Phys. 2020, 59. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.S.; Hong, S.H.; Hatta, A.; Short, R.D. Probing the Transport of Plasma-Generated RONS in an Agarose Target as Surrogate for Real Tissue: Dependency on Time, Distance and Material Composition. J. Phys. D Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical properties of amorphous Al2O3 thin films prepared by a sol–gel process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Beladiya, V.; Faraz, T.; Kessels, W.M.M.; Tünnermann, A.; Szeghalmi, A. Controlling mechanical, structural, and optical properties of Al2O3 thin films deposited by plasma-enhanced atomic layer deposition with substrate biasing. Proc. SPIE 2018, 10691, 106910. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Mistry, K.; Nguyen, V.H.; Ibrahim, K.H.; Muñoz-Rojas, D.; Yavuz, M.; Musselman, K.P. Quantum-Tunneling Metal-Insulator-Metal Diodes Made by Rapid Atmospheric Pressure Chemical Vapor Deposition. Adv. Funct. Mater. 2019, 29, 1805533. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS Characterization of ALD Al2O3 Coated Porous Silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]



| Activation Time (min) | pH | ORP (mV) | σ (µS/cm) | TDS (ppm) | RONS | ||||
|---|---|---|---|---|---|---|---|---|---|
| H2O2 (mg/L) | NO3− (mg/L) | NO2− (mg/L) | HNO2 (mg/L) | O3 (mg/L) | |||||
| 0 | 6.7 | 106 | 10 | 10 | - | - | - | - | - |
| 4 | 3.5 | 190 | 130 | 90 | 68.2 | 46.3 | 32.8 | 20.3 | 0.03 |
| 30 | 3.1 | 228 | 310 | 450 | 76.5 | 44.9 | 33.5 | 65.4 | 1.19 |
| 60 | 2.7 | 239 | 800 | 560 | 177.4 | 193.7 | 38.4 | 188.1 | >2.00 * |
| Sample | Oo/Al | Ot/Al | C% | Eg (±0.01 eV) |
|---|---|---|---|---|
| DI water | 1.66 | 1.82 | 3.60 | 5.97 |
| PAW 3.5 | 1.67 | 1.87 | 3.91 | 6.23 |
| PAW 3.1 | 1.56 | 1.81 | 3.42 | 6.09 |
| PAW 2.7 | 1.61 | 1.86 | 3.96 | 6.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, J.; Chiappim, W.; Karnopp, J.; Neto, B.; Leite, D.; da Silva Sobrinho, A.; Pessoa, R. Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth. Nanomaterials 2023, 13, 3110. https://doi.org/10.3390/nano13243110
Chaves J, Chiappim W, Karnopp J, Neto B, Leite D, da Silva Sobrinho A, Pessoa R. Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth. Nanomaterials. 2023; 13(24):3110. https://doi.org/10.3390/nano13243110
Chicago/Turabian StyleChaves, João, William Chiappim, Júlia Karnopp, Benedito Neto, Douglas Leite, Argemiro da Silva Sobrinho, and Rodrigo Pessoa. 2023. "Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth" Nanomaterials 13, no. 24: 3110. https://doi.org/10.3390/nano13243110
APA StyleChaves, J., Chiappim, W., Karnopp, J., Neto, B., Leite, D., da Silva Sobrinho, A., & Pessoa, R. (2023). Novel Energetic Co-Reactant for Thermal Oxide Atomic Layer Deposition: The Impact of Plasma-Activated Water on Al2O3 Film Growth. Nanomaterials, 13(24), 3110. https://doi.org/10.3390/nano13243110