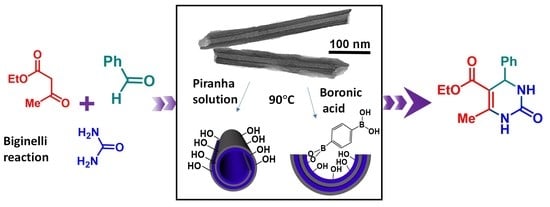
Halloysite Nanotubes as Bimodal Lewis/Brønsted Acid Heterogeneous Catalysts for the Synthesis of Heterocyclic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterizations
2.3. Procedures
- Grafting of boronic acid inside the lumen of HNTs
- Activation treatment of HNTS
- General procedure for the synthesis of 3,4-dihydropyrimidinones
3. Results and Discussion
3.1. Synthesis of Halloysite Nanotube-Based Acid Catalysts
3.2. Catalytic Performance of HNTs in the Biginelli Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. Solid acid catalysts. Curr. Opin. Solid State Mater. Sci. 1997, 2, 63–75. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Use of Solid Catalysts in Friedel-Crafts Acylation Reactions. Chem. Rev. 2006, 106, 1077–1104. [Google Scholar] [CrossRef]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Mondal, S.; Das, N.C. Mussel inspired green synthesis of silver nanoparticles-decorated halloysite nanotube using dopamine: Characterization and evaluation of its catalytic activity. Appl. Nanosci. 2018, 8, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Das, T.K.; Ghosh, S.; Ganguly, S.; Nath, K.; Das, N.C. Physico-mechanical, rheological and gas barrier properties of organoclay and inorganic phyllosilicate reinforced thermoplastic films. J. Appl. Polym. 2020, 138, 49735. [Google Scholar] [CrossRef]
- Abbasov, V.; Mammadova, T.; Aliyeva, N.; Abbasov, M.; Movsumov, N.; Joshi, A.; Lvov, Y.; Abdullayev, E. Catalytic cracking of vegetable oils and vacuum gasoil with commercial high alumina zeolite and halloysite nanotubes for biofuel production. Fuel 2016, 181, 55–63. [Google Scholar] [CrossRef]
- Silva, S.M.; Peixoto, A.F.; Freire, C. HSO3-functionalized halloysite nanotubes: New acid catalysts for esterification of free fatty acid mixture as hybrid feedstock model for biodiesel production. Appl. Catal. A Gen. 2018, 568, 221–230. [Google Scholar] [CrossRef]
- Mahajan, A.; Gupta, P. Halloysite nanotubes based heterogeneous solid acid catalysts. New J. Chem. 2020, 44, 12897–12908. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kravtsova, A.; Il’ina, I.; Wärnå, J.; Korchagina, D.; Gatilov, Y.V.; Volcho, K.; Salakhutdinov, N.; Murzin, D.Y.; Agabekov, V. Clay nanotubes catalyzed solvent-free synthesis of octahydro-2H-chromenols with pharmaceutical potential from (-)-isopulegol and ketones. J. Catal. 2019, 380, 145–152. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.; Mäki-Arvela, P.; Laluc, M.; Peixoto, A.F.; Kholkina, E.; Sandberg, T.; Aho, A.; Volcho, K.; Salakhutdinov, N.; Freire, C.; et al. Prins cyclization of (-)-isopulegol with benzaldehyde for production of chromenols over organosulfonic clays. Mol. Catal. 2019, 478, 110569. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kravtsova, A.V.; Aho, A.; Heinmaa, I.; Volcho, K.P.; Salakhutdinov, N.F.; Agabekov, V.E.; Murzin, D.Y. Acid-modified Halloysite Nanotubes as a Stereoselective Catalyst for Synthesis of 2H-Chromene Derivatives by the Reaction of Isopulegol with Aldehydes. ChemCatChem 2018, 10, 3950–3954. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, P.; Sharma, C. Surface modified novel magnetically tuned halloysite functionalized sulfonic acid: Synthesis, characterization and catalytic activity. Catal. Sci. Technol. 2021, 11, 3775–3786. [Google Scholar] [CrossRef]
- Samadani, M.; Asadi, B.; Mohammadpoor-Baltork, I.; Mirkhani, V.; Tangestaninejad, S.; Moghadam, M. Triazine bis(pyridinium) hydrogen sulfate ionic liquid immobillized on functionalized halloysite nanotubes as an efficient catalyst for one-pot synthesis of naphthopyranopyrimidines. RSC Adv. 2021, 11, 11976–11983. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.S.; Chowdhury, S. Recent developments in solvent-free multicomponent reactions: A perfect synergy for eco-compatible organic synthesis. RSC Adv. 2012, 2, 4547–4592. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Debache, A.; Boumoud, B.; Amimour, M.; Belfaitah, A.; Rhouati, S.; Carboni, B. Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction. Tetrahedron Lett. 2006, 47, 5697–5699. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, F.; Zhu, C. Highly Enantioseletive Biginelli Reaction Using a New Chiral Ytterbium Catalyst: Asymmetric Synthesis of Dihydropyrimidines. J. Am. Chem. Soc. 2005, 127, 16386–16387. [Google Scholar] [CrossRef] [PubMed]
- Sweet, F.; Fissekis, J.D. Synthesis of 3,4-dihydro-2(1H)-pyrimidinones and the mechanism of the Biginelli reaction. J. Am. Chem. Soc. 1973, 95, 8741–8749. [Google Scholar] [CrossRef]
- Paraskar, A.; Dewkar, G.; Sudalai, A. Cu(OTf)2: A reusable catalyst for high-yield synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2003, 44, 3305–3308. [Google Scholar] [CrossRef]
- Krishna, B.; Payra, S.; Roy, S. Synthesis of dihydropyrimidinones via multicomponent reaction route over acid functionalized Metal-Organic framework catalysts. J. Colloid Interface Sci. 2022, 607, 729–741. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Chen, Z.-Y.; Wei, N.; Liu, L.; Han, Z.-B. Highly Efficient Cooperative Catalysis of Single-Site Lewis Acid and Brønsted Acid in a Metal–Organic Framework for the Biginelli Reaction. Inorg. Chem. 2019, 58, 7657–7661. [Google Scholar] [CrossRef]
- Pal, T.K.; De, D.; Senthilkumar, S.; Neogi, S.; Bharadwaj, P.K. A Partially Fluorinated, Water-Stable Cu(II)–MOF Derived via Transmetalation: Significant Gas Adsorption with High CO2 Selectivity and Catalysis of Biginelli Reactions. Inorg. Chem. 2016, 55, 7835–7842. [Google Scholar] [CrossRef]
- Verma, A.; De, D.; Tomar, K.; Bharadwaj, P.K. An Amine Functionalized Metal–Organic Framework as an Effective Catalyst for Conversion of CO2 and Biginelli Reactions. Inorg. Chem. 2017, 56, 9765–9771. [Google Scholar] [CrossRef]
- Yao, B.-J.; Wu, W.-X.; Ding, L.-G.; Dong, Y.-B. Sulfonic Acid and Ionic Liquid Functionalized Covalent Organic Framework for Efficient Catalysis of the Biginelli Reaction. J. Org. Chem. 2021, 86, 3024–3032. [Google Scholar] [CrossRef]
- Allahresani, A.; Sangani, M.M.; Nasseri, M.A.; Hemmat, K. CoFe2O4@SiO2-NH2-CoII NPs: An effective magnetically recoverable catalyst for Biginelli reaction. Inorg. Chem. Commun. 2020, 118, 107988. [Google Scholar] [CrossRef]
- Yu, J.; Niedenthal, W.; Smarsly, B.M.; Natile, M.M.; Huang, Y.; Carraro, M. Au nanoparticles supported on piranha etched halloysite nanotubes for highly efficient heterogeneous catalysis. Appl. Surf. Sci. 2021, 546, 149100. [Google Scholar] [CrossRef]
- Zheng, H.; McDonald, R.; Hall, D.G. Boronic Acid Catalysis for Mild and Selective [3+2] Dipolar Cycloadditions to Unsaturated Carboxylic Acids. Chem. A Eur. J. 2010, 16, 5454–5460. [Google Scholar] [CrossRef]
- Sakakura, A.; Ohkubo, T.; Yamashita, R.; Akakura, M.; Ishihara, K. Brønsted Base-Assisted Boronic Acid Catalysis for the Dehydrative Intramolecular Condensation of Dicarboxylic Acids. Org. Lett. 2011, 13, 892–895. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic acid catalysis. Chem. Soc. Rev. 2019, 48, 3475–3496. [Google Scholar] [CrossRef]
- Liu, F.; Bai, L.; Zhang, H.; Song, H.; Hu, L.; Wu, Y.; Ba, X. Smart H2O2-Responsive Drug Delivery System Made by Halloysite Nanotubes and Carbohydrate Polymers. ACS Appl. Mater. Interfaces 2017, 9, 31626–31633. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Yu, J.; Boudjelida, S.; Galiano, F.; Figoli, A.; Bonchio, M.; Carraro, M. Porous Polymeric Membranes Doped with Halloysite Nanotubes and Oxygenic Polyoxometalates. Adv. Mater. Interfaces 2022, 9, 2102152. [Google Scholar] [CrossRef]
- Sun, P.; Liu, G.; Lv, D.; Dong, X.; Wu, J.; Wang, D. Effective activation of halloysite nanotubes by piranha solution for amine modification via silane coupling chemistry. RSC Adv. 2015, 5, 52916–52925. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Teraoka, S.; Matsushima, Y.; Kubo, Y. Fabrication of Soft Submicrospheres by Sequential Boronate Esterification and Their Dynamic Behavior. ChemPlusChem 2012, 77, 201–209. [Google Scholar] [CrossRef]
- Matsushima, Y.; Nishiyabu, R.; Takanashi, N.; Haruta, M.; Kimura, H.; Kubo, Y. Boronate self-assemblies with embedded Au nanoparticles: Preparation, characterization and their catalytic activities for the reduction of nitroaromatic compounds. J. Mater. Chem. 2012, 22, 24124–24131. [Google Scholar] [CrossRef]
- Breen, C.; D’Mello, N.; Yarwood, J. The thermal stability of mixed phenylphosphonic acid/water intercalates of kaolin and halloysite. A TG–EGA and VT-DRIFTS study. J. Mater. Chem. 2002, 12, 273–278. [Google Scholar] [CrossRef]
- Mahrez, N.; Bendenia, S.; Marouf-Khelifa, K.; Batonneau-Gener, I.; Khelifa, A. Improving of the adsorption capacity of halloysite nanotubes intercalated with dimethyl sulfoxide. Compos. Interfaces 2015, 22, 403–417. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Fialips, C.-I.; Vieillard, P.; Righi, D. Differences in the dehydration-rehydration behavior of halloysites: New evidence and interpretations. Clays Clay Miner. 2006, 54, 473–484. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, Y.; Sun, J.; Wang, Z. Thermal behavior analysis of halloysite–dimethylsulfoxide intercalation complex. J. Therm. Anal.Calorim. 2017, 129, 985–990. [Google Scholar] [CrossRef]
- Cromwell, B.; Levenson, A.; Levine, M. Thermogravimetric analysis of aromatic boronic acids for potential flame retardant applications. Thermochim. Acta 2019, 683, 178476. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Song, G.; Jia, H.; Shi, Z.; Liu, S.; Zhang, L.; Lin, J.; Nazarenko, S. Proton conductivity improvement of sulfonated poly(ether ether ketone) nanocomposite membranes with sulfonated halloysite nanotubes prepared via dopamine-initiated atom transfer radical polymerization. J. Membr. Sci. 2016, 504, 206–219. [Google Scholar] [CrossRef]
- Zhao, Z.; Ran, J.; Jiao, Y.; Li, W.; Miao, B. Modified natural halloysite nanotube solely employed as an efficient and low-cost solid acid catalyst for alpha-arylstyrenes production via direct alkenylation. Appl. Catal. A Gen. 2016, 513, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, K.; An, Z.; Zhu, Y.; Shu, X.; Song, H.; Xiang, X.; He, J. Acid–Base Promoted Dehydrogenation Coupling of Ethanol on Supported Ag Particles. Ind. Eng. Chem. Res. 2020, 59, 3342–3350. [Google Scholar] [CrossRef]
- Che, M. Nobel Prize in chemistry 1912 to Sabatier: Organic chemistry or catalysis? Catal. Today 2013, 218–219, 162–171. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science. Dev. Clay Sci. 2006, 1, 1–18. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kravtsova, A.; Wärnå, J.; Aho, A.; Heinmaa, I.; Il’ina, I.; Ardashov, O.; Volcho, K.; Salakhutdinov, N.; Murzin, D.Y.; et al. Preparation of octahydro-2H-chromen-4-ol with analgesic activity from isopulegol and thiophene-2-carbaldehyde in the presence of acid-modified clays. Mol. Catal. 2018, 453, 139–148. [Google Scholar] [CrossRef]
- Sidera, M.; Fletcher, S. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem. 2015, 7, 935–939. [Google Scholar] [CrossRef]
- Zheng, H.; Ghanbari, S.; Nakamura, S.; Hall, D.G. Boronic Acid Catalysis as a Mild and Versatile Strategy for Direct Carbo- and Heterocyclizations of Free Allylic Alcohols. Angew. Chem. Int. Ed. 2012, 51, 6187–6190. [Google Scholar] [CrossRef]
- Letsinge, R.L.; Dandegaonker, S.; Vullo, W.S.; Morrison, J. Organoboron Compounds. XIV.1,2 Polyfunctional Catalysis by 8-Quinolineboronic Acid. J. Am. Chem. Soc. 1963, 85, 2223–2227. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Q.; Liu, J.; Zhang, P.; Fu, S.; Wu, S. Iron–Organic Framework Nanoparticle-Supported Tungstosilicic Acid as a Catalyst for the Biginelli Reaction. ACS Appl. Nano Mater. 2022, 5, 16987–16995. [Google Scholar] [CrossRef]
- Quan, Z.J.; Da, Y.X.; Zhang, Z.; Wang, X.C. PS–PEG–SO3H as an efficient catalyst for 3, 4-dihydropyrimidones via Biginelli reaction. Catal. Commun. 2019, 10, 1146–1148. [Google Scholar] [CrossRef]
- Konkala, K.; Sabbavarapu, N.M.; Katla, R.; Durga, N.Y.V.; Reddy, V.K.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N. Revisit to the Biginelli reaction: A novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3, 4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett. 2012, 53, 1968–1973. [Google Scholar] [CrossRef]
- Yao, N.; Lu, M.; Liu, X.B.; Tan, J.; Hu, Y.L. Copper-doped mesoporous silica supported dual acidic ionic liquid as an efficient and cooperative reusability catalyst for Biginelli reaction. J. Mol. Liq. 2018, 262, 328–335. [Google Scholar] [CrossRef]






 HNTs catalyzed Biginelli reaction—Reaction scope | ||||
 |  |  |  |  |
| 4a Yield 71 % | 4b Yield 46 % | 4c Yield 70 % | 4d Yield 65 % | 4e Yield 54 % |
 |  |  |  |  |
| 4f Yield 64 % | 4g Yield 68 % | 4h Yield 56 % | 4i Yield 62 % | 4j Yield 69 % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Mateos, J.; Carraro, M. Halloysite Nanotubes as Bimodal Lewis/Brønsted Acid Heterogeneous Catalysts for the Synthesis of Heterocyclic Compounds. Nanomaterials 2023, 13, 394. https://doi.org/10.3390/nano13030394
Yu J, Mateos J, Carraro M. Halloysite Nanotubes as Bimodal Lewis/Brønsted Acid Heterogeneous Catalysts for the Synthesis of Heterocyclic Compounds. Nanomaterials. 2023; 13(3):394. https://doi.org/10.3390/nano13030394
Chicago/Turabian StyleYu, Jiaying, Javier Mateos, and Mauro Carraro. 2023. "Halloysite Nanotubes as Bimodal Lewis/Brønsted Acid Heterogeneous Catalysts for the Synthesis of Heterocyclic Compounds" Nanomaterials 13, no. 3: 394. https://doi.org/10.3390/nano13030394
APA StyleYu, J., Mateos, J., & Carraro, M. (2023). Halloysite Nanotubes as Bimodal Lewis/Brønsted Acid Heterogeneous Catalysts for the Synthesis of Heterocyclic Compounds. Nanomaterials, 13(3), 394. https://doi.org/10.3390/nano13030394