Nanoporous Hollow Carbon Spheres Derived from Fullerene Assembly as Electrode Materials for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FE-HS and Carbonized FE-HS
2.3. Characterizations
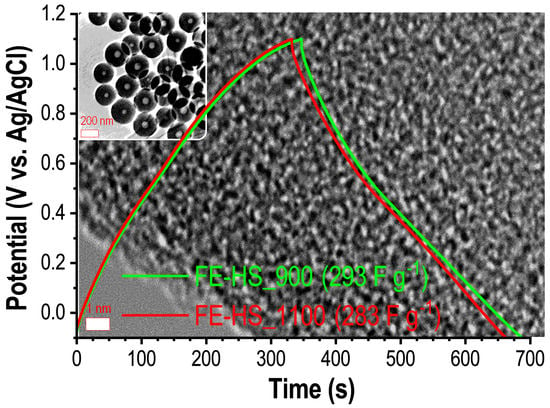
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Sing, G.; Gopalan, A.-I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent advances in functionalized nanoporous carbons derived from waste resources and their applications in energy and environment. Adv. Sustain. Syst. 2021, 5, 2000169. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics of nanoporous carbon materials in supercapacitors applications. Nanomaterials 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Tang, J.; Zhang, W.; Zhang, K.; Chen, Y.; Gao, R.; Yin, H.; Yu, X.; Qin, L.-C. Facile preparation of flexible binder-free graphene electrodes for high-performance supercapacitors. RSC Adv. 2022, 12, 12590–12599. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Rawson, T.J.; Belyustina, M.A. Designing supercapacitor electrolyte via ion counting. Energy Environ. Sci. 2022, 15, 2948–2957. [Google Scholar] [CrossRef]
- Chen, W.; Yang, K.; Luo, M.; Zhang, D.; Li, Z.; Liu, C.; Zhou, X. Carbonization-free wood electrode with MXene-reconstructed porous structure for all-wood eco-supercapacitors. EcoMat 2023, 5, e12271. [Google Scholar] [CrossRef]
- Hao, L.; Ning, J.; Luo, B.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, J.; Thomas, A.; Zhi, L. Structural evolution of 2D microporous covalent triazine-based framework toward the study of high-performance supercapacitors. J. Am. Chem. Soc. 2015, 137, 219–225. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Ilbeygi, H.; Park, D.-H.; Sarkar, S.; Chandra, G.; Umapathy, S.; Srinivasan, S.; Talapaneni, S.; Vinu, A. Highly crystalline mesoporous C60 with ordered pores: A class of nanomaterials for energy applications. Angew. Chem. Int. Ed. 2018, 57, 569–573. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Baskar, A.V.; Park, D.-H.; Chandra, G.; Umapathy, S.; Talapaneni, N.; Vinu, A. Ordered mesoporous C70 with highly crystalline pore walls for energy applications. Adv. Funct. Mater. 2018, 28, 1803701. [Google Scholar] [CrossRef]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous carbon cubes derived from fullerene crystals as a high rate performance electrode materials for supercapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, K.; Mao, Y.; Liu, C.; Pang, M.; Li, H. Nanoarchitectonics of mesoporous carbon from C60/PCBM hybrid crystals for supercapacitor. Carbon 2023, 201, 449–459. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Ji, Q.; Mori, T.; Miyazawa, K.; Yamauchi, Y.; Hill, J.P.; Ariga, K. Fullerene nanoarchitectonics: From zero to higher dimension. Chem. Asian J. 2013, 8, 1662–1679. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Tsuruoka, T.; Acharya, S.; Ji, Q.; Hill, J.P.; Ariga, K.; Yamauchi, Y.; Shrestha, L.K. Mesoporous fullerene C70 cubes with highly crystalline frameworks and unusually enhanced photoluminescence properties. Mater. Horiz. 2018, 5, 285–290. [Google Scholar] [CrossRef]
- Zheng, S.; Coung, N.T.; Okada, S.; Xu, T.; Shen, W.; Lu, X.; Tsukagoshi, K. Solvent-mediated shape engineering of fullerene (C60) polyhedral microcrystals. Chem. Mater. 2018, 30, 7145–7153. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, X.; Guan, Y.; Wang, Y.; Jin, F.; Guan, R.; Liu, F.; Chen, M.; Tian, Y.; Yang, S. From cubes to dice: Solvent-regulated morphology engineering of endohedral fullerene microcrystals with anomalous photoluminescence enhancement. Angew. Chem. Int. Ed. 2019, 58, 11350–11354. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, R.; Feng, Y.; Wang, S.; Liu, L.; Li, X.; Han, Y.; Chen, H. On demand synthesis of hollow fullerene nanostructures. Nat. Commun. 2019, 10, 1548. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Tang, Q.; Zhao, W.; Sun, J.; An, R.; Niu, T.; Fuchs, H.; Ji, Q. Tailoring structural features and functions of fullerene rod crystals by a ferrocene-modified fullerene derivative. CrystEngComm 2020, 22, 6287–6294. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsu, S.-H.; Maji, S.; Chahal, M.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2020, 7, 787–795. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Zero-to-one (or more) nanoarchitectonics: How to produce functional materials from zero-dimensional single-element unit, fullerene. Mater. Adv. 2021, 2, 582–597. [Google Scholar] [CrossRef]
- Baskar, A.V.; Benzigar, M.R.; Talapaneni, S.N.; Singh, G.; Karakoti, A.S.; Yi, J.; Al-Muhtaseb, A.H.; Ariga, K.; Ajayan, P.M.; Vinu, A. Self-assembled fullerene nanostructures: Synthesis and applications. Adv. Funct. Mater. 2022, 32, 2106924. [Google Scholar] [CrossRef]
- Chen, G.; Bhadra, B.N.; Sutrisno, L.; Shrestha, L.K.; Ariga, K. Fullerene rosette: Two-dimensional interactive nanoarchitectonics and selective vapor sensing. Int. J. Mol. Sci. 2022, 23, 5454. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sciortino, F.; Takeyashu, K.; Nakamura, J.; Hill, J.P.; Shrestha, L.K.; Ariga, K. Hollow spherical fullerene obtained by kinetically controlled liquid-liquid interfacial precipitation. Chem. Asian J. 2022, 17, e202200756. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Tang, M.; Wu, Y.; Chen, Y.; Zhu, S.; Wang, Q.; Xia, C.; Wang, C. Size control of zwitterionic polymer micro/nanospheres and its dependence on sodium storage. Nanoscale Horiz. 2019, 4, 1092–1098. [Google Scholar] [CrossRef]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, O.; Hahn, M.; Herzog, A.; Kötz, R. Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 2005, 43, 1303–1310. [Google Scholar] [CrossRef]
- You, X.; Misra, M.; Gregori, S.; Mohanty, A.K. Preparation of an electric double layer capacitor (EDLC) using miscanthus-derived biocarbon. ACS Sustain. Chem. Eng. 2018, 6, 318–324. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspective for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, D.; Minakshi, M.; Quadsia, S.; Ahuja, R. Activation-induced surface modulation of biowaste-derived hierarchical porous carbon for supercapacitors. ChemPlusChem 2022, 87, e202200126. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous carbon tubes from fullerene crystals as the π-electron carbon source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Tsuruoka, T.; Ji, Q.; Nishimura, T.; Ariga, K. Surfactant-triggered nanoarchitectonics of fullerene C60 crystals at a liquid−liquid interface. Langmuir 2016, 32, 12511–12519. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D mesoporous carbon microbelts derived from fullerene crystals as an electrode material for electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465. [Google Scholar] [CrossRef]
- Zheng, S.; Ju, H.; Lu, X. A High-Performance Supercapacitor Based on KOH Activated 1D C70 Microstructures. Adv. Energy Mater. 2015, 5, 1500871. [Google Scholar] [CrossRef]
- Bairi, P.; Shrestha, R.G.; Hill, J.P.; Nishimura, T.; Ariga, K.; Shrestha, L.K. Mesoporous graphitic carbon microtubes derived from fullerene C70 tubes as a high performance electrode materials for advanced supercapacitors. J. Mater. Chem. A 2016, 4, 13899–13906. [Google Scholar] [CrossRef] [Green Version]
- Seaton, N.A.; Walton, J.P.R.B.; Quirke, N. A new analysis for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Hirsch, A.; Li, Q.; Wudl, F. Globe-trotting hydrogens on the surface of the fullerene compound C60H6(N(CH2CH2)2O)6. Angew. Chem. Int. Ed. Engl. 1991, 30, 1309–1310. [Google Scholar] [CrossRef]
- Isobe, H.; Tanaka, T.; Nakanishi, W.; Lemiègre, L.; Nakamura, E. Regioselective oxygenative tetraamination of [60]fullerene. fullerene-mediated reduction of molecular oxygen by amine via ground state single electron transfer in dimethyl Sulfoxide. J. Org. Chem. 2005, 70, 4826–4832. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, Z.; Jia, Z.; Huang, S.; Yang, X.; Li, Y.; Gan, L.; Zhang, S.; Zhu, D. Amination of [60]fullerene by ammonia and by primary and secondary aliphatic amines—Preparation of amino[60]fullerene peroxides. Chem. Eur. J. 2007, 13, 1129–1141. [Google Scholar] [CrossRef]
- Matsuoka, K.; Akiyama, T.; Yamada, S. Selective formation and structural properties of rhombic dodecahedral [70]fullerene microparticles formed by reaction with aliphatic diamines. Langmuir 2010, 26, 4274–4280. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, C.; Liu, C.; Liu, J.; Zhu, Y.; Wang, X. Nitrogen-doped hollow carbon spheres derived from amination reaction of fullerene with alkyl diamines as a carbon catalyst for hydrogenation of aromatic nitro compounds. Carbon 2017, 125, 139–145. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, Z.; Dong, J.; Yao, M.; Yang, Z.; Li, Q.; Zahi, C.; Shen, F.; Hou, X.; Wang, L.; et al. Ultrahard bulk amorphous fullerene. Nature 2021, 599, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, Y.; Piao, G.; Guldi, D.M. Control over tuning fullerene microcrystals by means of engineering charge-transfer interactions. ACS Appl. Mater. Interfaces 2019, 11, 16567–16570. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Xu, S.-A. Preparation and fire behavior of rigid polyurethane foams synthesized from modified urea–melamine–formaldehyde resins. RSC Adv. 2018, 8, 17879–17887. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Golden, T.C.; Sircar, S. Activated carbon adsorbent for PSA driers. Carbon 1990, 28, 683–690. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Perez-Ramirez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Schneider, P.; Hudec, P.; Solcova, O. Pore-volume and surface area in microporous–mesoporous solids. Microporous Mesoporous Mater. 2008, 115, 491–496. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Qi, G.; Li, B.; Song, Z.; Jinag, H.; Zhang, X.; Qiao, J. A novel N-doped porous carbon microspheres composed of hollow carbon nanospheres. Carbon 2016, 96, 864–870. [Google Scholar] [CrossRef]
- Mehra, P.; Singh, C.; Cherian, I.; Giri, A.; Paul, A. Diciphering the incredible supercapacitor performance conducting biordered ultramicroporous graphitic carbon. ACS Appl. Energy Mater. 2021, 4, 4416–4427. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanism for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef] [Green Version]
- Jäckel, N.; Simon, P.; Gogotsi, Y.; Presser, V. Increase in capacitance by subnanometer pores in carbon. ACS Energy Lett. 2016, 1, 1262–1265. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Chen, Z.; Tian, Q.; Cao, Y.; Wang, C.; Liu, Y.; Fu, J.; Zhang, J.; Zhu, L.; Yang, C.; et al. Synthesis of uniform discrete cage-like nitrogen-doped hollow porous carbon spheres with tunable direct large mesoporous for ultrahigh supercapacitive performance. Appl. Surf. Sci. 2017, 425, 69–76. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of Nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Bang, J.-H.; Lee, B.-H.; Choi, Y.-C.; Lee, H.-M.; Kim, B.-J. A study on superior mesoporous activated carbons for ultra powder density supercapacitor from biomass precursors. Int. J. Mol. Sci. 2022, 23, 8537. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, Q.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Wei, J.; Luo, C.; Li, H.; Lv, W.; Liang, J.; Deng, Y.; Huang, Z.; Wang, C.; Kang, F.; Yang, Q.-H. Direct assembly of micron-size porous graphene spheres with a high density as supercapacitor materials. Carbon 2019, 149, 492–498. [Google Scholar] [CrossRef]
- Chen, S.; Ma, W.; Cheng, Y.; Weng, Z.; Sun, B.; Wang, L.; Chen, W.; Li, F.; Zhu, M.; Cheng, H.-M. Scalable non-liquid-crystal spinning of locally aligned graphene fibers for high-performance wearable supercapacitors. Nano Energy 2015, 15, 642–653. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Müllen, K. Three-dimensional graphene-based macro- and mesoporous frameworks for high-performance electrochemical capacitive energy storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Yang, H.; Wang, L.; Teh, B.K.; Zhong, J.; Chou, H.; Chen, L.; Shen, Z.; Ruoff, R.S.; Lin, J. Preparation of supercapacitor electrodes through selection of graphene surface functionalities. ACS Nano 2012, 6, 5941–5951. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Nady, J.E.; Wazeer, W.; Kashyout, A.B. Development of high-performance supercapacitor based on a novel controllable green synthesis for 3D nitrogen doped graphene. Sci. Rep. 2019, 9, 1129. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mi, R.; Yuan, L.; Yang, F.; Fu, Z.; Wang, C.; Tang, Y. Nitrogen-doped multi-scale porous carbon for high voltage aqueous supercapacitors. Front. Chem. 2018, 6, 475. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Tian, C.; Fu, Y.; Yang, Y.; Yin, J.; Wang, L.; Fu, H. Nitrogen-doped porous graphitic carbon as an excellent electrode material for advanced supercapacitors. Chem. Eur. J. 2014, 20, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, I.-W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Ambade, R.B.; Noh, S.H.; Eom, W.; Koh, K.H.; Ambade, S.B.; Lee, W.J.; Kim, S.H.; Han, T.H. Porous graphene-carbon nanotube scaffolds for fiber supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 9011–9022. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, M.; Zhou, X.; Li, J.; Dong, Y.; Zhu, M. Three-dimensional porous carbon nanotubes/reduced graphene oxide fiber from rapid phase separation for a high-rate all-solid-state supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 9283–9290. [Google Scholar] [CrossRef]
- Tan, Z.; Ni, K.; Chen, G.; Zeng, W.; Tao, Z.; Ikram, M.; Zhang, Q.; Wang, H.; Sun, L.; Zhu, X.; et al. Incorporating pyrrolic and pyridinic nitrogen into a porous carbon made from C60 molecules to obtain superior energy storage. Adv. Mater. 2017, 29, 1603414. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, R.G.; Lee, J.; Han, S.A.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Macaroni Fullerene Crystals-Derived Mesoporous Carbon Tubes as a High Rate Performance Supercapacitor Electrode Material. Bull. Chem. Soc. Jpn. 2021, 94, 1502–1509. [Google Scholar] [CrossRef]








| Sample | SSA (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vp (cm3 g−1) | Vmicro (cm3 g−1) | Wp (nm) | Dp (nm) |
|---|---|---|---|---|---|---|---|
| FE-HS | 94.2 | 42.4 | 51.8 | 0.530 | 0.154 | --- | --- |
| FE-HS_700 | 612.9 | 499.4 | 113.5 | 0.925 | 0.350 | 0.299 | 3.89 |
| FE-HS_900 | 1616.5 | 1504.3 | 112.2 | 1.295 | 0.655 | 0.262 | 3.89 |
| FE-HS_1100 | 1439.3 | 1264.2 | 175.1 | 1.346 | 0.630 | 0.274 | 3.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, L.K.; Wei, Z.; Subramaniam, G.; Shrestha, R.G.; Singh, R.; Sathish, M.; Ma, R.; Hill, J.P.; Nakamura, J.; Ariga, K. Nanoporous Hollow Carbon Spheres Derived from Fullerene Assembly as Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2023, 13, 946. https://doi.org/10.3390/nano13050946
Shrestha LK, Wei Z, Subramaniam G, Shrestha RG, Singh R, Sathish M, Ma R, Hill JP, Nakamura J, Ariga K. Nanoporous Hollow Carbon Spheres Derived from Fullerene Assembly as Electrode Materials for High-Performance Supercapacitors. Nanomaterials. 2023; 13(5):946. https://doi.org/10.3390/nano13050946
Chicago/Turabian StyleShrestha, Lok Kumar, Zexuan Wei, Gokulnath Subramaniam, Rekha Goswami Shrestha, Ravi Singh, Marappan Sathish, Renzhi Ma, Jonathan P. Hill, Junji Nakamura, and Katsuhiko Ariga. 2023. "Nanoporous Hollow Carbon Spheres Derived from Fullerene Assembly as Electrode Materials for High-Performance Supercapacitors" Nanomaterials 13, no. 5: 946. https://doi.org/10.3390/nano13050946
APA StyleShrestha, L. K., Wei, Z., Subramaniam, G., Shrestha, R. G., Singh, R., Sathish, M., Ma, R., Hill, J. P., Nakamura, J., & Ariga, K. (2023). Nanoporous Hollow Carbon Spheres Derived from Fullerene Assembly as Electrode Materials for High-Performance Supercapacitors. Nanomaterials, 13(5), 946. https://doi.org/10.3390/nano13050946