The Influence of Carbonaceous Matrices and Electrocatalytic MnO2 Nanopowders on Lithium-Air Battery Performances
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Structural Characterization of MnO2 Nanomaterials

| Sample | SBET (m2·g−1) | Vtot. pores (mL·g−1) | 6 nm < d < 20 nm |
|---|---|---|---|
| M_hydro | 97 | 0.336 | 49 |
| M_200 | 70 | 0.391 | 40 |
| M_300 | 61 | 0.360 | 39 |
| M_400 | 46 | 0.310 | 19 |
| M_500 | 28 | 0.290 | 2 |
| M_hydro_0.5%Ag | 88 | 0.325 | 51 |
| M_hydro_1.0%Ag | 75 | 0.300 | 50 |
| M_hydro_2.0%Ag | 73 | 0.248 | 49 |



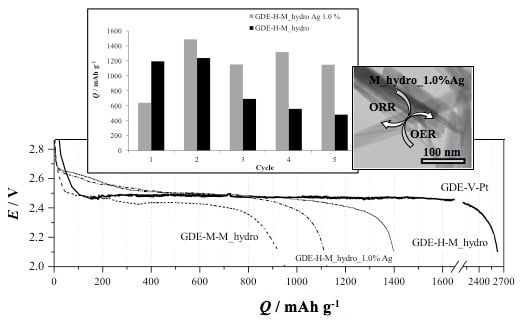
2.2. Electrochemical Characterization of GDEs
| Name | Composition | Loading (mg·cm−2) |
|---|---|---|
| GDE-V | PVDF (15%), SAB (20%), Vulcan XC72R (65%) | 1.3 |
| GDE-V-Pt | PVDF (15%), SAB (20%), 10% Pt-loaded Vulcan XC72R (65%) | 1.3 |
| GDE-M | PVDF (15%), SAB (20%), MCC (65%) | 1.1 |
| GDE-M-M_hydro | PVDF (15%), SAB (20%), MCC (45%), M_hydro (20%) | 2.2 |
| GDE-H | PVDF (15%), SAB (20%), HCMSC (65%) | 1.4 |
| GDE-H-M_hydro | PVDF (15%), SAB (20%), HCMSC (45%), M_hydro (20%) | 1.3 |
| GDE-H-M_hydro_1.0%Ag | PVDF (15%), SAB (20%), HCMSC (45%), M_hydro_1.0%Ag (20%) | 1.3 |




3. Experimental Section
3.1. Cathode Material and Gas Diffusion Electrode
3.2. Cell Configuration
3.3. Instrumentation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Minguzzi, A.; Locatelli, C.; Cappelletti, G.; Bianchi, C.L.; Vertova, A.; Ardizzone, S.; Rondinini, S. Designing materials by means of the cavity-microelectrode: The introduction of the quantitative rapid screening toward a highly efficient catalyst for water oxidation. J. Mater. Chem. 2012, 22, 8896–8902. [Google Scholar] [CrossRef]
- Minguzzi, A.; Locatelli, C.; Lugaresi, O.; Vertova, A.; Rondinini, S. Au-based/electrochemically etched cavity-microelectrodes as optimal tool for quantitative analyses on finely dispersed electrode materials: Pt/C, IrO2-SnO2 and Ag catalysts. Electrochim. Acta 2013, 114, 637–642. [Google Scholar] [CrossRef]
- Locatelli, C.; Minguzzi, A.; Vertova, A.; Cava, P.; Rondinini, S. Quantitative studies on electrode material properties by means of the cavity microelectrode. Anal. Chem. 2011, 83, 2819–2823. [Google Scholar] [CrossRef] [PubMed]
- Minguzzi, A.; Lugaresi, O.; Locatelli, C.; Rondinini, S.; D’Acapito, F.; Achilli, E.; Ghigna, P. Fixed energy X-ray absorption voltammetry. Anal. Chem. 2013, 85, 7009–7013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Foster, D.; Read, J. Discharge characteristic of a non-aqueous electrolyte Li/O2 battery. J. Power Sources 2010, 195, 1235–1240. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Gasteiger, H.A.; Crumlin, E.; McGuire, R.; Shao-Horn, Y. Electrocatalytic Activity Studies of Select Metal Surfaces and Implications in Li-Air Batteries. J. Electrochem. Soc. 2010, 157, A1016–A1025. [Google Scholar] [CrossRef]
- Hassoun, J.; Croce, F.; Armand, M.; Scrosati, B. Investigation of the O2 electrochemistry in a polymer electrolyte solid-state cell. Angew. Chem. Int. Ed. 2011, 50, 2999–3002. [Google Scholar] [CrossRef] [PubMed]
- Padbury, R.; Zhang, X. Lithium–oxygen batteries—Limiting factors that affect performance. J. Power Sources 2011, 196, 4436–4444. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. A review of high energy density lithium-air battery technology. J. Appl. Electrochem. 2014, 44, 5–22. [Google Scholar] [CrossRef]
- Cheng, H.; Scott, K. Nano-structured gas diffusion electrode—A high power and stable cathode material for rechargeable Li-air batteries. J. Power Sources 2013, 235, 226–233. [Google Scholar] [CrossRef]
- Calegaro, M.L.; Lima, F.H.B.; Ticianelli, E.A. Oxygen reduction reaction on nanosized manganese oxide particles dispersed on carbon in alkaline solutions. J. Power Sources 2006, 158, 735–739. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Ji, S.; Goh, J.; Feng, H.; Wang, R. Highly active Vulcan carbon composite for oxygen reduction reaction in alkaline medium. Electrochim. Acta 2014, 133, 391–398. [Google Scholar] [CrossRef]
- Tran, C.; Yang, X.Q.; Qu, D. Investigation of the gas-diffusion-electrode used as lithium/air cathode in non-aqueous electrolyte and the importance of carbon material porosity. J. Power Sources 2010, 195, 2057–2063. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, D.; Xu, W.; Wang, D.; Williford, R.E.; Liu, J.; Zhang, J.-G. Optimization of Air Electrode for Li/Air Batteries. J. Electrochem. Soc. 2010, 157, A487–A492. [Google Scholar] [CrossRef]
- Débart, A.; Bao, J.; Armstrong, G.; Bruce, P.G. An O2 cathode for rechargeable lithium batteries: The effect of a catalyst. J. Power Sources 2007, 174, 1177–1182. [Google Scholar] [CrossRef]
- Locatelli, C.; Minguzzi, A.; Vertova, A.; Rondinini, S. IrO2–SnO2 mixtures as electrocatalysts for the oxygen reduction reaction in alkaline media. J. Appl. Electrochem. 2013, 43, 171–179. [Google Scholar] [CrossRef]
- Minguzzi, A.; Locatelli, C.; Cappelletti, G.; Scavini, M.; Vertova, A.; Ghigna, P.; Sandra Rondinini, S. IrO2-based disperse-phase electrocatalysts: A complementary study by means of the cavity-microelectrode and ex-situ X-ray absorption spectroscopy. J. Phys. Chem. A 2012, 116, 6497–6504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Fontaine, O.; Bruce, P.G. Charging a Li-O2 battery using a redox mediator. Nat. Chem. 2013, 5, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.D.; Song, H.; Kim, J.; Gwon, H.; Bae, Y.; Park, K.Y.; Hong, J.; Kim, H.; Kim, T.; Kim, Y.H.; et al. Superior rechargeability and efficiency of lithium-oxygen batteries: Hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. 2014, 53, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shen, Y.; Zhang, W.; Yu, L.; Yi, Z.; Yin, W.; Wang, D.; Huang, Y.; Wang, J.; Wang, D.; et al. A solution-phase bifunctional catalyst for lithium-oxygen batteries. J. Am. Chem. Soc. 2014, 136, 8941–8946. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Scott, K. Selection of oxygen reduction catalysts for rechargeable lithium-air batteries-metal or oxide? Appl. Catal. B 2011, 108–109, 140–151. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Cho, M.-S.; Yun, K.-S.; Kim, T.-S.; Park, C.-J. Catalytic characteristics of MnO2 nanostructures for the O2 reduction process. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Viswanathan, V.V.; Wang, D.; Towne, S.A.; Xiao, J.; Nie, Z.; Hu, D.; Zhang, J.G. Investigation on the charging process of Li2O2-based air electrodes in Li-O2 batteries with organic carbonate electrolytes. J. Power Sources 2011, 196, 3894–3899. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Chen, Y.; Peng, Z.; Griffin, J.M.; Hardwick, L.J.; Bardé, F.; Novák, P.; Bruce, P.G. Reactions in the rechargeable lithium-O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 2011, 133, 8040–8047. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, F.; Xiao, J.; Zhang, J.; Xu, W.; Park, S.; Zhang, J.G.; Wang, Y.; Liu, J. Making Li-air batteries rechargeable: Material challenges. Adv. Funct. Mater. 2013, 23, 987–1004. [Google Scholar] [CrossRef]
- Bardenhagen, I.; Fenske, M.; Fenske, D.; Wittstock, A.; Bäumer, M. Distribution of discharge products inside of the lithium/oxygen battery cathode. J. Power Sources 2015, 299, 162–169. [Google Scholar] [CrossRef]
- Bardenhagen, I.; Yezerska, O.; Augustin, M.; Fenske, D.; Wittstock, A.; Bäumer, M. In situ investigation of pore clogging during discharge of a Li/O2 battery by electrochemical impedance spectroscopy. J. Power Sources 2015, 278, 255–264. [Google Scholar] [CrossRef]
- Marchini, F.; Herrera, S.; Torres, W.; Tesio, A.Y.; Williams, F.J.; Calvo, E.J. Surface Study of Lithium–Air Battery Oxygen Cathodes in Different Solvent–Electrolyte Pairs. Langmuir 2015, 31, 9236–9245. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, M.; Balasubramanian, P.; Gucciardi, E.; Theil, S.; Jçrissen, L. Importance of Reaction Kinetics and Oxygen Crossover in aprotic Li-O2 Batteries Based on a Dimethyl Sulfoxide Electrolyte. ChemSusChem 2015, 8, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, Y.J. Effect of multi-catalysts on rechargeable Li-air batteries. J. Alloys Compd. 2014, 591, 164–169. [Google Scholar] [CrossRef]
- Ogasawara, T.; Débart, A.; Holzapfel, M.; Novák, P.; Bruce, P.G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Débart, A.; Paterson, A.J.; Bao, J.; Bruce, P.G. α-MnO2 nanowires: A catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 4521–4524. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Buseck, P.R. Defects in nsutite (γ-MnO2) and dry-cell battery efficiency. Nature 1983, 304, 143–146. [Google Scholar] [CrossRef]
- De Wolff, P.M. Interpretation of some γ-MnO2 diffraction patterns. Acta Crystallogr. 1959, 12, 341–345. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and electrochemical properties of the proton/γ-MnO2 system. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Benhaddad, L.; Makhloufi, L.; Messaoudi, B.; Rahmouni, K.; Takenouti, H. Reactivity of Nanostructured MnO2 in Alkaline Medium Studied with a Micro-Cavity Electrode: Effect of Synthesizing Temperature. ACS Appl. Mater. Interfaces 2009, 1, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, Y.; Chiba, H.; Atou, T.; Kikuchi, M.; Hiraga, K.; Syono, Y. Preparation of γ-MnO2 with an Open Tunnel. J. Solid State Chem. 1999, 144, 136–142. [Google Scholar] [CrossRef]
- Ching, S.; Petrovay, D.J.; Jorgensen, M.L.; Suib, S.L. Sol−Gel Synthesis of Layered Birnessite-Type Manganese Oxides. Inorg. Chem. 1997, 36, 883–890. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol-gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Byström, A.M.; Lund, E.W.; Lund, L.K.; Hakala, M. The Crystal Structure of Ramsdellite, an Orthorhombic Modification of MnO2. Acta Chem. Scand. 1949, 3, 163–173. [Google Scholar] [CrossRef]
- Baur, W.H. Rutile-type compounds. V. Refinement of MnO2 and MgF2. Acta Crystallogr. Sect. B 1976, 32, 2200–2204. [Google Scholar] [CrossRef]
- Post, J.E.; von Dreele, R.B.; Buseck, P.R. Symmetry and cation displacements in hollandites: Structure refinements of hollandite, cryptomelane and priderite. Acta Crystallogr. Sect. B 1982, 38, 1056–1065. [Google Scholar] [CrossRef]
- Geller, S. Structure of α-Mn2O3, (Mn0.983Fe0.017)2O3 and (Mn0.37Fe0.63)2O3 and relation to magnetic ordering. Acta Crystallogr. Sect. B 1971, 27, 821–828. [Google Scholar] [CrossRef]
- Joo, J.B.; Kim, P.; Kim, W.; Kim, J.; Yi, J. Preparation of mesoporous carbon templated by silica particles for use as a catalyst support in polymer electrolyte membrane fuel cells. Catal. Today 2006, 111, 171–175. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.-H.; Luo, Z.-K.; Pang, Y.; Wu, Q.-X.; Liang, C.-S.; Chen, J.; Liu, D.; Zhang, X. A dual pore carbon aerogel based air cathode for a highly rechargeable lithium-air battery. J. Power Sources 2014, 272, 1061–1071. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, T.; Ozcan, S.; Uysal, M.; Guler, M.O.; Akbulut, H. Free-standing flexible graphene oxide paper electrode for rechargeable Li-O2 batteries. J. Power Sources 2014, 267, 140–147. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Gasteiger, H.A.; Parent, M.C.; Chiloyan, V.; Shao-Horn, Y. The Influence of Catalysts on Discharge and Charge Voltages of Rechargeable Li-Oxygen Batteries. Electrochem. Solid-State Lett. 2010, 13, A69–A72. [Google Scholar] [CrossRef]
- Du, Z.; Yang, P.; Wang, L.; Lu, Y.; Goodenough, J.B.; Zhang, J.; Zhang, D. Electrocatalytic performances of LaNi1−xMgxO3 perovskite oxides as bi-functional catalysts for lithium air batteries. J. Power Sources 2014, 265, 91–96. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Huang, G.; Wang, J.; Du, X.; Qin, Y.; Wang, L. Freestanding MnO2@carbon papers air electrodes for rechargeable Li-O2 batteries. J. Power Sources 2014, 261, 311–316. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Shen, Y.-W.; Chien, L.-H.; Kuo, P.-L. Li2FeSiO4 nanorod as high stability electrode for lithium-ion batteries. J. Nanoparticle Res. 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Shao-Horn, Y. Electrocatalytic Measurement Methodology of Oxide Catalysts Using a Thin-Film Rotating Disk Electrode. J. Electrochem. Soc. 2010, 157, B1263–B1268. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Ionita, M.; Minguzzi, A.; Rondinini, S.; Vertova, A. Composite ternary SnO2-IrO2-Ta2O5 oxide electrocatalysts. J. Electroanal. Chem. 2006, 589, 160–166. [Google Scholar] [CrossRef]
- Ionita, M.; Cappelletti, G.; Minguzzi, A.; Ardizzone, S.; Bianchi, C.; Rondinini, S.; Vertova, A. Bulk, Surface and Morphological Features of Nanostructured Tin Oxide by a Controlled Alkoxide-Gel Path. J. Nanoparticle Res. 2006, 8, 653–660. [Google Scholar] [CrossRef]
- Benhaddad, L.; Bazin, C.; Makhloufi, L.; Messaoudi, B.; Pillier, F.; Rahmouni, K.; Takenouti, H. Effect of synthesis duration on the morphological and structural modification of the sea urchin-nanostructured γ-MnO2 and study of its electrochemical reactivity in alkaline medium. J. Solid State Electrochem. 2014, 18, 2111–2121. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, L.; Zhang, M.; Guo, S.; Liu, Z.H.; Yang, Z.; Wang, Z. Preparation of Ag-nanoparticle-loaded MnO2 nanosheets and their capacitance behavior. Energy Fuels 2012, 26, 618–623. [Google Scholar] [CrossRef]
- Zeng, J.; Francia, C.; Dumitrescu, M.A.; Monteverde Videla, A.H.A.; Ijeri, V.S.; Specchia, S.; Spinelli, P. Electrochemical performance of Pt-based catalysts supported on different ordered mesoporous carbons (Pt/OMCs) for oxygen reduction reaction. Ind. Eng. Chem. Res. 2012, 51, 7500–7509. [Google Scholar] [CrossRef]
- Büchel, G.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. A Novel Pathway for Synthesis of Submicrometer-Size Solid Core/Mesoporous Shell Silica Spheres. Adv. Mater. 1998, 10, 1036–1038. [Google Scholar] [CrossRef]
- Zeng, J.; Francia, C.; Gerbaldi, C.; Dumitrescu, M.A.; Specchia, S.; Spinelli, P. Smart synthesis of hollow core mesoporous shell carbons (HCMSC) as effective catalyst supports for methanol oxidation and oxygen reduction reactions. J. Solid State Electrochem. 2012, 16, 3087–3096. [Google Scholar] [CrossRef]
- Maja, M.; Orecchia, C.; Strano, M.; Tosco, P.; Vanni, M. Effect of structure of the electrical performance of gas diffusion electrodes for metal air batteries. Electrochim. Acta 2000, 46, 423–432. [Google Scholar] [CrossRef]
- Beattie, S.D.; Manolescu, D.M.; Blair, S.L. High-Capacity Lithium-Air Cathodes. J. Electrochem. Soc. 2009, 156, A44–A47. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef] [Green Version]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minguzzi, A.; Longoni, G.; Cappelletti, G.; Pargoletti, E.; Di Bari, C.; Locatelli, C.; Marelli, M.; Rondinini, S.; Vertova, A. The Influence of Carbonaceous Matrices and Electrocatalytic MnO2 Nanopowders on Lithium-Air Battery Performances. Nanomaterials 2016, 6, 10. https://doi.org/10.3390/nano6010010
Minguzzi A, Longoni G, Cappelletti G, Pargoletti E, Di Bari C, Locatelli C, Marelli M, Rondinini S, Vertova A. The Influence of Carbonaceous Matrices and Electrocatalytic MnO2 Nanopowders on Lithium-Air Battery Performances. Nanomaterials. 2016; 6(1):10. https://doi.org/10.3390/nano6010010
Chicago/Turabian StyleMinguzzi, Alessandro, Gianluca Longoni, Giuseppe Cappelletti, Eleonora Pargoletti, Chiara Di Bari, Cristina Locatelli, Marcello Marelli, Sandra Rondinini, and Alberto Vertova. 2016. "The Influence of Carbonaceous Matrices and Electrocatalytic MnO2 Nanopowders on Lithium-Air Battery Performances" Nanomaterials 6, no. 1: 10. https://doi.org/10.3390/nano6010010
APA StyleMinguzzi, A., Longoni, G., Cappelletti, G., Pargoletti, E., Di Bari, C., Locatelli, C., Marelli, M., Rondinini, S., & Vertova, A. (2016). The Influence of Carbonaceous Matrices and Electrocatalytic MnO2 Nanopowders on Lithium-Air Battery Performances. Nanomaterials, 6(1), 10. https://doi.org/10.3390/nano6010010