Repair of the Orbital Wall Fractures in Rabbit Animal Model Using Nanostructured Hydroxyapatite-Based Implant
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
| Sample Type | Ca (%) | P (%) | Ca:P Ratio | BET Specific Surface Area (m2/g) | BJH 2 Adsorption Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| HAp nanopowder | 39.34 | 19.15 | 1.59 | 37.11 | 11.80 |
| HAp sintered at 800 °C/30 min | 39.80 | 19.30 | 1.60 | 13.95 | 8.16 |
2.2. Structural Characterization (X-Ray Diffraction)

2.3. Morphological Characterization (SEM Analysis)

2.4. Particle Size Distribution

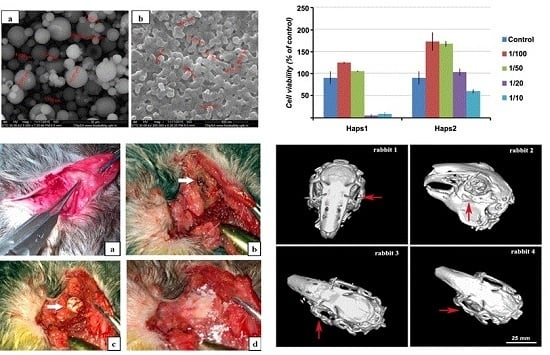
2.5. In Vitro Test

2.6. In Vivo Test
| Male Rabbit | Maximum Density of the Implant One Month Postoperative (HU) | Maximum Density of the Implant Two Months Postoperative (HU) | Normal Orbital Density (HU) |
| Rabbit 1 | 202 | 400 | 900 |
| Rabbit 2 | 315 | 450 | 1000 |
| Rabbit 3 | 250 | 402 | 1120 |
| Rabbit 4 | 309 | 409 | 940 |
| Male Rabbit | Maximum Density of the Surrounding Fibrovascular Tissue One Month Postoperative (HU) | Maximum Density of the Surrounding Fibrovascular Tissue Two Months Postoperative (HU) | Normal Orbital Density (HU) |
| Rabbit 1 | 40 | 70 | 900 |
| Rabbit 2 | 50 | 72 | 1000 |
| Rabbit 3 | 40 | 75 | 1120 |
| Rabbit 4 | 60 | 70 | 940 |





3. Experimental Section
3.1. Hydrothermal Synthesis of NanoHAp Powder
3.2. Manufacturing of NanoHAp-Based Sintered Pellets
3.3. Characterization of NanoHAp
3.3.1. Physico-Chemical Characterization
3.3.2. Structural Characterization (XRD Analysis)
3.3.3. Morphological Characterization (SEM Analysis)
3.3.4. Particle Size Distribution
3.4. Evaluation of Biocompatibility by In Vitro Cell Culture Studies
3.5. In Vivo Test
3.5.1. Animal model
3.5.2. Surgical Intervention Methodology

3.5.3. Postoperative Care
- (1)
- Topogram (selection of the region of interest).
- (2)
- Initial acquisition of 2.4 (avoiding movement artifacts by shortening the exposure and acquisition time).
- (3)
- Reconstruction (HeadSecv 1.2, Headsecv 0.6 and SSD (3D), frontal and sagittal multiplanar reconstruction).
- (4)
- Hounsfield units assessment of the implant and related tissues.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schon, R.; Metzger, M.C.; Zizelmann, C.; Weyer, N.; Schmelzeisen, R. Individually preformed titanium mesh implants for a true-to-original repair of orbital fractures. Int. J. Oral Max. Surg. 2006, 35, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komunski, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefanczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio Maxill. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Walkowiak, B.; Stefanczyk, L. Technical concept of patient-specific, ultrahigh molecular weight polyethylene orbital wall implant. J. Cranio Maxill. Surg. 2013, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Sinikovic, B.; Kramer, F.J.; Swennen, G.; Lubbers, H.T.; Dempf, R. Reconstruction of orbital wall defects with calcium phosphate cement: Clinical and histological findings in a sheep model. Int. J. Oral Max. Surg. 2007, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Treatment with individual orbital wall implants in humans—1-Year ophthalmologic evaluation. J. Cranio Maxill. Surg. 2011, 39, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Loba, P.; Kozakiewicz, M.; Elgalal, M.; Stefanczyk, L.; Broniarczyk-Loba, A.; Omulecki, W. The use of modern imaging techniques in the diagnosis and treatment planning of patients with orbital floor fractures. Med. Sci. Monit. 2011, 17, CS94–CS98. [Google Scholar] [CrossRef]
- He, D.; Li, Z.; Shi, W.; Sun, Y.; Zhu, H.; Lin, M.; Shen, G.; Fan, X. Orbitozygomatic fractures with enophthalmos: Analysis of 64 cases treated late. J. Oral Maxil. Surg. 2012, 70, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Fricia, M.; Passanisi, M.; Salamanna, F.; Parrilli, A.; Giavaresi, G.; Fini, M. Osteointegration in custom-made porous hydroxyapatite cranial implants: From reconstructive surgery to regenerative medicine. World Neurosurg. 2015, 84. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliver. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; McArdle, A.; Atashroo, D.; Walmsley, G.G.; Senarath-Yapa, K.; Zielins, E.R.; Paik, K.J.; Longaker, M.T.; Wan, D.C. Biomaterials for craniofacial bone engineering. J. Dent. Res. 2014, 93, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Wen, Z.; Yang, S.; Wang, J.; Zhang, Q. Controllable self-assembly of mesoporous hydroxyapatite. Colloids Surf. B 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, F.; Salavati-Niasari, M.; Fathi, M.; Fereshteh, Z. Hydroxyapatite nanocrystals: Simple preparation, characterization and formation mechanism. Mat. Sci. Eng. C 2014, 45, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zogbi, M.M., Jr.; Saito, E.; Zanin, H.; Marciano, F.R.; Lobo, A.O. Hydrothermal–electrochemical synthesis of nano-hydroxyapatite crystals on superhydrophilic vertically aligned carbon nanotubes. Mater. Lett. 2014, 132, 70–74. [Google Scholar] [CrossRef]
- Liu, T.; Ding, X.; Yang, X.; Gou, Z.; Chen, J.; Feng, X. Effect of carbonization treatment on the morphology and microstructure of mesoporous bioactive glass/nanocrystalline hydroxyapatite nanocomposite. J. Non-Cryst. Solids 2014, 389, 104–112. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Wang, Z.; Li, Y.; Ran, H.; Niu, L.; Gong, P.; Liu, B.; Yang, S. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014, 66, 407–416. [Google Scholar] [CrossRef]
- Lin, K.; Liu, P.; Wei, L.; Zou, Z.; Zhang, W.; Qian, Y.; Shen, Y.; Chang, J. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. 2013, 222, 49–59. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Costa, E.; Santos-Silva, A.; Pintado, M.M.E.; Castro, P.M.L. Hydroxyapatite-based materials of marine origin: A bioactivity and sintering study. Mater. Sci. Eng. C 2015, 51, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Scientific Comkittee on Consumer Safety (SCCS). Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/index_en.htm (accessed on 16 October 2015).
- Shuai, C.; Feng, P.; Cao, C.; Peng, S. Processing and Characterization of Laser Sintered Hydroxyapatite. Biotechnol. Bioprocess Eng. 2013, 18, 520–527. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Cheng, Y.; Wang, L.; Hu, B.; Tan, J. Effects of hydrothermal temperature and time on hydrothermal synthesis of colloidal hydroxyapatite nanorods in the presence of sodium citrate. J. Colloid Interf. Sci. 2015, 450, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, J.; Feng, Y.; Liu, J.; Yu, X. Synthesis and characterization of ultra long nanofibrillar and hydroxyapatite powder. Adv. Powder Technol. 2015, 26, 428–433. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Murakami, S.; Kato, K.; Enari, Y.; Kamitakahara, M.; Watanabe, N.; Ioku, K. Hydrothermal synthesis of porous hydroxyapatite ceramics composed of rod-shaped particles and evaluation of their fracture behavior. Ceram. Int. 2012, 38, 1649–1654. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Y.; Cai, Y.; Yao, J. Effect of amino acids on the crystal growth of hydroxyapatite. Mater. Lett. 2012, 68, 361–363. [Google Scholar] [CrossRef]
- Gao, S.; Sun, K.; Li, A.; Wang, H. Synthesis and characterization of hydroxyapatite nanofiber by chemical precipitation method using surfactants. Mater. Res. Bull. 2013, 48, 1003–1006. [Google Scholar] [CrossRef]
- Yang, X.; Gao, X.; Gan, Y.; Gao, C.; Zhang, X.; Ting, K.; Wu, B.M.; Gou, Z. Facile Synthesis of Octacalcium Phosphate Nanobelts: Growth Mechanism and Surface Adsorption Properties. J. Phys. Chem. C 2010, 114, 6265–6271. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Low-temperature synthesis of nanoparticle-assembled, transparent, and low-crystallized hydroxyapatite blocks. J. Colloid Interf. Sci. 2011, 360, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Layrolle, P.; Ito, A.; Tateishi, T. Sol–Gel Synthesis of Amorphous Calcium Phosphate and Sintering into Microporous Hydroxyapatite Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1421–1428. [Google Scholar] [CrossRef]
- Bakan, F.; Laçin, O.; Sarac, H. A novel low temperature sol-gel synthesis process for thermally stable nano crystalline hydroxyapatite. Powder Technol. 2013, 233, 295–302. [Google Scholar] [CrossRef]
- Popescu, L.M.; Piticescu, R.M.; Rusti, C.F.; Maly, M.; Danani, A.; Kintzios, S.; Valero Grinan, M.T. Preparation and characterization of new hybrid nanostructured thin films for biosensors design. Mater. Lett. 2011, 65, 2032–2035. [Google Scholar] [CrossRef]
- Popescu, L.M.; Rusti, C.F.; Piticescu, R.M.; Buruiana, T.; Valero, T.; Kintzios, S. Synthesis and characterization of acid polyurethane—Hydroxyapatite composites for biomedical applications. J. Compos. Mater. 2013, 47, 603–612. [Google Scholar] [CrossRef]
- Vasile, E.; Popescu, L.M.; Piticescu, R.M.; Burlacu, A.; Buruiana, T. Physico-chemical and biocompatible properties of hydroxyapatite based composites prepared by an innovative synthesis route. Mater. Lett. 2012, 79, 85–88. [Google Scholar] [CrossRef]
- Popescu, L.M.; Piticescu, R.M.; Antonelli, A.; Rusti, C.F.; Carboni, E.; Sfara, C.; Magnani, M.; Badilita, V.; Vasile, E.; Trusca, R.; et al. Recent advances in synthesis, characterization of hydroxyapatite/polyurethane composites and study of their biocompatible properties. J. Mater. Sci. Mater. Med. 2013, 24, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.L.; Piticescu, R.M.; Petrescu, Ş.; Zdrenţu, L.; Mihăilescu, I.; Socol, G.; Lojkowski, W. Biocompatibility of hydroxyl-apatite thin films obtained by pulsed laser deposition. Rev. Adv. Mater. Sci. 2004, 8, 164–169. [Google Scholar]
- Williams, J.S.; Xiao, Y.; Brownell, I. Low pH reprograms somatic murine cells into pluripotent stem cells: A novel technique with therapeutic implications. Cancer Biol. Ther. 2014, 15, 675–677. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradinaru, S.; Popescu, L.M.; Piticescu, R.M.; Zurac, S.; Ciuluvica, R.; Burlacu, A.; Tutuianu, R.; Valsan, S.-N.; Motoc, A.M.; Voinea, L.M. Repair of the Orbital Wall Fractures in Rabbit Animal Model Using Nanostructured Hydroxyapatite-Based Implant. Nanomaterials 2016, 6, 11. https://doi.org/10.3390/nano6010011
Gradinaru S, Popescu LM, Piticescu RM, Zurac S, Ciuluvica R, Burlacu A, Tutuianu R, Valsan S-N, Motoc AM, Voinea LM. Repair of the Orbital Wall Fractures in Rabbit Animal Model Using Nanostructured Hydroxyapatite-Based Implant. Nanomaterials. 2016; 6(1):11. https://doi.org/10.3390/nano6010011
Chicago/Turabian StyleGradinaru, Sinziana, Laura Madalina Popescu, Roxana Mioara Piticescu, Sabina Zurac, Radu Ciuluvica, Alexandrina Burlacu, Raluca Tutuianu, Sorina-Nicoleta Valsan, Adrian Mihail Motoc, and Liliana Mary Voinea. 2016. "Repair of the Orbital Wall Fractures in Rabbit Animal Model Using Nanostructured Hydroxyapatite-Based Implant" Nanomaterials 6, no. 1: 11. https://doi.org/10.3390/nano6010011
APA StyleGradinaru, S., Popescu, L. M., Piticescu, R. M., Zurac, S., Ciuluvica, R., Burlacu, A., Tutuianu, R., Valsan, S. -N., Motoc, A. M., & Voinea, L. M. (2016). Repair of the Orbital Wall Fractures in Rabbit Animal Model Using Nanostructured Hydroxyapatite-Based Implant. Nanomaterials, 6(1), 11. https://doi.org/10.3390/nano6010011