Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Hybrid Nanostructures
2.3. Characterization of the Solutions and Hybrid Nanostructures
2.4. Contact Angle and Surface Free Energy Measurements
2.5. In Vitro Drug Release Study
3. Results and Discussion
3.1. Characterization of the Hybrid Nanostructures
3.2. Contact Angle and Surface Free Energy Measurements
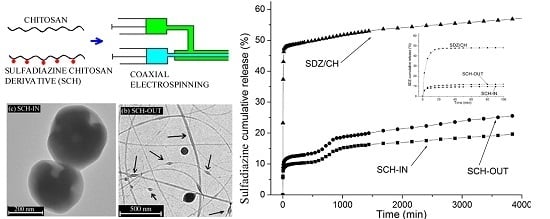
3.3. In Vitro Drug Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Qiu, Z. Fluid bed coating and granulation for CR delivery. In Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wen, H., Park, K., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2010; p. 117. [Google Scholar]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, G.; Hu, H.; Yang, J.; Qian, B.; Ling, Z.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon nanofiber/graphene composite electrode for supercapacitors. J. Power Sources 2013, 243, 350–353. [Google Scholar] [CrossRef]
- Bae, H.; Lee, J. Encapsulated particles attached on electrospun fibers by in situ combination of electrospinning and coaxial electrospraying. J. Nanosci. Nanotechnol. 2014, 14, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Xuyen, N.T.; Kim, T.H.; Geng, H.Z.; Lee, I.H.; Kim, K.K.; Lee, Y.H. Three-dimensional architecture of carbon nanotube-anchored polymer nanofiber composite. J. Mater. Chem. 2009, 19, 7822–7825. [Google Scholar] [CrossRef]
- Vitchuli, N.; Shi, Q.; Nowak, J.; Kay, K.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. Multifunctional ZnO/Nylon 6 nanofiber mats by an electrospinning-electrospraying hybrid process for use in protective applications. Sci. Technol. Adv. Mater. 2011, 12, 055004. [Google Scholar] [CrossRef]
- Krupa, A.; Jaworek, A.; Sundarrajan, S.; Pliszka, D.; Ramakrishna, S. Mechanical properties of an electrospun polymer fibre-metal oxide nanocomposite mat. Fibres Text. East. Eur. 2012, 20, 25–27. [Google Scholar]
- Avila, H.A.; Reboredo, M.M.; Castro, M.; Parra, R. Nanofibers obtained by electrospinning of BaTiO3 particles dispersed in polyvinyl alcohol and ethylcellulose. Mater. Res. 2013, 16, 839–843. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Zamboni, F.; Acasigua, G.A.; Pranke, P. Association of electrospinning with electrospraying: A strategy to produce 3D scaffolds with incorporated stem cells for use in tissue engineering. Int. J. Nanomed. 2015, 10, 5159–5169. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Venugopal, J.; Mitra, S.; Giri Dev, V.R.; Ramakrishna, S. Nanostructured biocomposite substrates by electrospinning and electrospraying for the mineralization of osteoblasts. Biomaterials 2009, 30, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly(l-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A.; Lackowski, M.; Sobczyk, A.T.; Czech, T.; Ramakrishna, S.; Sundarrajan, S.; Pliszka, D. Nanocomposite fabric formation by electrospinning and electrospraying technologies. J. Electrost. 2009, 67, 435–438. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; De Bank, P.A. Electrospun matrices for localised controlled drug delivery: Release of tetracycline hydrochloride from layers of polycaprolactone and poly(ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2012, 2, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.I.; Lau, Y.C.; Yan, C.; Coombes, A.G.A. Controlled release of an antibiotic, gentamicin sulphate, from gravity spun polycaprolactone fibers. J. Biomed. Mater. Res. A 2008, 84, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonov, V.V.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.P.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Hilmi, A.B.M.; Halim, A.; Hassan, A.; Lim, C.K.; Noorsal, K.; Zainol, I. In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. SpringerPlus 2013, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. B 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, A.; Hoeksema, H.; Vandekerckhove, D.; Pirayesh, A.; Monstrey, S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016, 42, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Cuttle, L.; Pearn, J.; McMillan, J.R.; Kimble, R.M. A review of first aid treatments for burn injuries. Burns 2009, 35, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Shariatinia, Z.; Pourmortazavi, S.M. Antibacterial electrospun chitosan–polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr. Polym. 2016, 140, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Moon, J.H.; Park, H.N.; Ko, W.K.; Bae, M.S.; Lee, J.B.; Park, S.W.; Kim, E.C.; Lee, C.H.; et al. Chitosan/polyurethane blended fiber sheets containing silver sulfadiazine for use as an antimicrobial wound dressing. J. Nanosci. Nanotechnol. 2014, 14, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, C.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Multilayer sodium alginate beads with porous core containing chitosan based nanoparticles for oral delivery of anticancer drug. Int. J. Biol. Macromol. 2016, 85, 1–8. [Google Scholar]
- Bodmeier, R.; Paeratakul, R. Spherical agglomerates of water-insoluble drugs. J. Pharm. Sci. 1989, 78, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, P.; Martins, J.T.; Fucinos, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Panzariu, A.T.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitriu, R.P.; Profire, L.; Nita, L.E.; Dragostin, O.M.; Ghetu, N.; Pieptu, D.; Vasile, C. Sulfadiazine-chitosan conjugates and their polyelectrolyte complexes with hyaluronate destined to the management of burn wounds. Materials 2015, 8, 317–338. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Tripatanasuwan, S.; Zhong, Z.; Reneker, D.H. Effect of evaporation and solidification of the charged jet in electrospinning of poly(ethylene oxide) aqueous solution. Polymer 2007, 48, 5742–5746. [Google Scholar] [CrossRef]
- Pascu, M.C.; Popescu, M.C.; Vasile, C. Surface modifications of some nanocomposites containing starch. J. Phys. D 2008, 41, 175407–175419. [Google Scholar] [CrossRef]
- Albu, R.M.; Avram, E.; Stoica, I.; Ioanid, E.G.; Popovici, D.; Ioan, S. surface properties and compatibility with blood of new quaternized polysulfones. J. Biomater. Nanobiotechnol. 2011, 2, 114–124. [Google Scholar] [CrossRef]
- Dobos, A.M.; Onofrei, M.D.; Ioan, S. Liquid crystals and cellulose derivatives composites. In Green Biorenewable Biocomposites: From Knowledge to Industrial Applications; Thakur, V.K., Kessler, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015; p. 101. [Google Scholar]
- Vijayanand, K.; Pattanayak, D.K.; Rama Mohan, T.R.; Banerjee, R. Interpreting blood-biomaterial interactions from surface free energy and work of adhesion. Trends Biomater. Artif. Organs 2005, 18, 73–83. [Google Scholar]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A.C.S.C. Photochemical degradation of sulfadiazine, sulfamerazine and sulfamethazine: Relevance of concentration and heterocyclicaromatic groups to degradation kinetics. J. Photochem. Photobiol. A 2014, 286, 40–46. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Lustig, S.R.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers I: Mathematical modeling. J. Polym. Sci. B 1986, 24, 395–408. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ceccone, G.; Gilliland, D.; Kulisch, W. Surface analytical characterization of biosenzor materials. In Nanotechnological Basis for Advanced Sensors; Reithmaier, J.P., Paunovic, P., Kulisch, W., Popov, C., Petkov, P., Eds.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Bhaskara Rao, S.; Chandra Sharma, P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar]
- Brink, G.H.; Foley, N.; Zwaan, D.; Kooi, B.J.; Palasantzas, G. Roughness controlled superhydrophobicity on single nanometer length scale with metal nanoparticles. RSC Adv. 2015, 5, 28696–28702. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Nambab, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influence implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [PubMed]
- Leung, V.; Hartwell, R.; Yang, H.; Ghahary, A.; Ko, F. Bioactive nanofibers for wound healing applications. J. Fiber. Bioeng. Inform. 2011, 4, 1–14. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis (Review). Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef] [PubMed]
- Nageh, H.; Ezzat, M.; Ghanim, M.; Hassanin, A.; El-Moneim, A.A. Evaluation of antibacterial activity and drug release behavior of chitosan-based nanofibers (In Vitro Study). UK J. Pharm. Biosci. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Liu, H.; Leonas, K.K.; Zhao, Y. Antimicrobial properties and release profile of ampicillin from electrospun poly(ε-caprolactone) nanofiber yarns. J. Eng. Fiber Fabr. 2010, 5, 10–19. [Google Scholar]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Effect of biphasic calcium phosphates on drug release and biological and mechanical properties of poly(ε-caprolactone) composite membranes. J. Biomed. Mater. Res. A 2004, 70, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids. Surf. B 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.P.; Saldanha, T.D.P.; Navarro, M.V.M.; Medeiros, A.C.; Ginani, M.F.; Raffin, F.N. Mechanical properties and release studies of chitosan films impregnated with silver sulfadiazine. J. Appl. Polym. Sci. 2006, 102, 3462–3470. [Google Scholar] [CrossRef]
- Dutta, P.K.; Khatua, M.K.; Dutta, J.; Prasad, R. Use of chitosan-DMAc/LiCl gel as drug carriers. Int. J. Chem. Sci. 2003, 1, 93–102. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Argin, S.; Kofinas, P.; Martin Lo, Y. The cell release kinetics and the swelling behavior of physically crosslinked xanthanechitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Ge, Y.; Mei, Z.; Liu, X. Evaluation of Daidzein-loaded chitosan microspheres In Vivo after intramuscular injection in rats. Yakugaku Zasshi 2011, 131, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Shapera, R.M. Meningococcal colonization and infection in children and their household contacts. Am. J. Epidemiol. 1979, 109, 563–571. [Google Scholar]
- Schifman, R.B.; Ryan, K.J. Neisseria lactamica Septicemia in an Immunocompromised Patient. J. Clin. Microbiol. 1983, 17, 934–935. [Google Scholar] [PubMed]
- Bakkali, N.; Fenollar, F.; Rolain, J.M.; Raoult, D. Comment on: Therapy for Whipple’s disease. J. Antimicrob. Chemother. 2008, 61, 968–969. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Value |
|---|---|
| Feed rate (for both the sulfadiazine modified chitosan (SCH) and chitosan (CH) solution) | 0.7 µL/min |
| Needle-collector voltage | 15 kV |
| Needle to collector distance | 9 cm |
| Deposition time | 120 min |
| Sample | Average Particle Sizes (nm) | Average Fiber Diameter (nm) |
|---|---|---|
| SCH and CH separately electrospun through the inner and outer nozzle respectively (SCH-IN) | 40 ± 10 (narrow distribution). Also, there are large particles (up to 300 nm) | 32 ± 10 |
| SCH and CH separately electrospun through the outer and inner nozzle respectively (SCH-OUT) | 35 ± 10 | 30 ± 10 |
| chitosan/sulfadiazine mixture (CH/SDZ) electrospun alone. | 40 ± 10 | 35 ± 10 |
| Sample | C (at %) | O (at %) | N (at %) | S (at %) |
|---|---|---|---|---|
| SCH-IN | 64.1 | 30.7 | 4.9 | 0.2 |
| SCH-OUT | 68 | 26.6 | 5 | 0.4 |
| SDZ/CH | 60.9 | 30.8 | 7.8 | 0.5 |
| Samples | γsvLW | γsvab | γsv+ | γsv− | γsvTOT | Ws/rbc | Ws/p |
|---|---|---|---|---|---|---|---|
| SDZ/CH | 43.5 | 3.4 | 3.3 | 0.9 | 47.0 | 30.3 | −88.3 |
| SCH-OUT | 26.4 | 10.6 | 6.9 | 4.0 | 37.0 | 24.2 | −105.6 |
| SCH-IN | 30.1 | 1.1 | 0.07 | 4.7 | 31.2 | −4.1 | −110.5 |
| Sample | M∞ (%) (after 65 h) | b (%) | n | k (h−1) | R2 |
|---|---|---|---|---|---|
| SCH-IN | 20 | 1/2 | 2.13 ± 0.05 | 0.04 | 0.99 ± 0.06 |
| SCH-OUT | 25 | 13/25 | 1.84 ± 0.08 | 0.1 | 0.99 ± 0.09 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, B.S.; Dumitriu, R.P.; Profire, L.; Sacarescu, L.; Hitruc, G.E.; Stoleru, E.; Dobromir, M.; Matricala, A.L.; Vasile, C. Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers. Nanomaterials 2016, 6, 207. https://doi.org/10.3390/nano6110207
Munteanu BS, Dumitriu RP, Profire L, Sacarescu L, Hitruc GE, Stoleru E, Dobromir M, Matricala AL, Vasile C. Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers. Nanomaterials. 2016; 6(11):207. https://doi.org/10.3390/nano6110207
Chicago/Turabian StyleMunteanu, Bogdanel Silvestru, Raluca Petronela Dumitriu, Lenuta Profire, Liviu Sacarescu, Gabriela Elena Hitruc, Elena Stoleru, Marius Dobromir, Ana Lavinia Matricala, and Cornelia Vasile. 2016. "Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers" Nanomaterials 6, no. 11: 207. https://doi.org/10.3390/nano6110207
APA StyleMunteanu, B. S., Dumitriu, R. P., Profire, L., Sacarescu, L., Hitruc, G. E., Stoleru, E., Dobromir, M., Matricala, A. L., & Vasile, C. (2016). Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers. Nanomaterials, 6(11), 207. https://doi.org/10.3390/nano6110207