Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of ZnO Nanostructures
2.2. Material Characterization Details
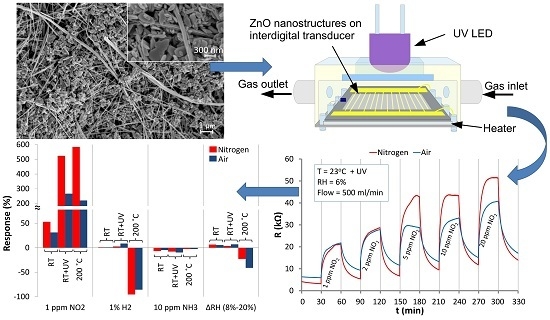
2.3. Fabrication of Resistive Structures Based on ZnO Nanostructures
2.4. Gas Measurement Details
2.5. Details Concerning Spectral Measurements
2.6. Details Concerning the Temperature Measurements
3. Results and Discussion
3.1. ZnO Structures Characterization
3.2. Investigations of the Influence of Light on the Resistance of ZnO Nanostructures
3.3. The Effect of the Carrier Gas and Temperature on the Electrical Properties of ZnO Nanostructures
3.4. The Reaction of ZnO Nanostructures to NO2
3.5. Reactions of ZnO Nanostructures with Reducing Gases
3.5.1. The Reaction of ZnO Nanstructures with Hydrogen
3.5.2. The Reaction of ZnO Nanostructures with Ammonia
3.6. The Response of ZnO Nanostructures to Changes of the Humidity
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Zhai, T.; Bando, Y.; Golberg, D. Recent progress of one-dimensional ZnO nanostructured solar cells. Nano Energy 2012, 1, 91–106. [Google Scholar] [CrossRef]
- Kayaci, F.; Vempati, S.; Donmez, I.; Biyikli, N.; Uyar, T. Role of zinc interstitials and oxygen vacancies of ZnO in photocatalysis: A bottom-up approach to control defect density. Nanoscale 2014, 6, 10224. [Google Scholar] [CrossRef] [PubMed]
- Cun, T.; Dong, C.; Huang, Q. Ionothermal precipitation of highly dispersive ZnO nanoparticles with improved photocatalytic performance. Appl. Surf. Sci. 2016, 384, 73–82. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, G.; Wang, J.; Xian, F.; Liu, Y. Stable co-emission of UV, green and red light in ZnO thin films with rapid annealing treatment. Opt. Int. J. Light Electron Opt. 2016, 127, 5942–5949. [Google Scholar] [CrossRef]
- Saoud, F.S.; Plenet, J.C.; Henini, M. Band gap and partial density of states for ZnO: Under high pressure. J. Alloys Compd. 2015, 619, 812–819. [Google Scholar] [CrossRef]
- Ojha, A.K.; Srivastava, M.; Kumar, S.; Hassanein, R.; Singh, J.; Singh, M.K.; Materny, A. Influence of crystal size on the electron–phonon coupling in ZnO nanocrystals investigated by Raman spectroscopy. Vib. Spectrosc. 2014, 72, 90–96. [Google Scholar] [CrossRef]
- Morkoc, H.; Ozgur, U. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. High-sensitivity NO2 gas sensors based on flower-like and tube-like ZnO nanomaterials. Sens. Actuators B 2011, 157, 565–574. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, A.; Huang, L.; Li, C.; Shi, G. High-performance NO2 sensors based on chemically modified graphene. Adv. Mater. 2013, 25, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Fanni, L.; Aebersold, B.A.; Alexander, D.T.L.; Ding, L.; Morales Masis, M.; Nicolay, S.; Ballif, C. c-texture versus a-texture low pressure metalorganic chemical vapor deposition ZnO films: Lower resistivity despite smaller grain size. Thin Solid Films 2014, 565, 1–6. [Google Scholar] [CrossRef]
- Rai, P.; Khan, R.; Ahmad, R.; Hahn, Y.-B.; Lee, I.-H.; Yu, Y.-T. Gas sensing properties of single crystalline ZnO nanowires grown by thermal evaporation technique. Curr. Appl. Phys. 2013, 13, 1769–1773. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Xiong, H.; Dai, J. Controlled synthesis of ZnO hollow microspheres via precursor-template method and its gas sensing property. Appl. Surf. Sci. 2012, 258, 8431–8438. [Google Scholar] [CrossRef]
- Bai, S.; Sun, C.; Guo, T.; Luo, R.; Lin, Y.; Chen, A.; Sun, L.; Zhang, J. Low temperature electrochemical deposition of nanoporous ZnO thin films as novel NO2 sensors. Electrochim. Acta 2013, 90, 530–534. [Google Scholar] [CrossRef]
- Thareja, R.K.; Saxena, H.; Narayanan, V. Laser-ablated ZnO for thin films of ZnO and MgxZn(1−x)O. J. Appl. Phys. 2005, 98, 34908. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T.; Krysztafkiewicz, A. Obtaining zinc oxide from aqueous solutions of KOH and Zn(CH3COO)2. Physicochem. Probl. Miner. Process. 2010, 44, 93–102. [Google Scholar]
- Tong, Y.; Liu, Y.; Dong, L.; Zhao, D.; Zhang, J.; Lu, Y.; Shen, D.; Fan, X. Growth of ZnO nanostructures with different morphologies by using hydrothermal technique. J. Phys. Chem. B 2006, 110, 20263–20267. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Pustelny, T.; Procek, M.; Maciak, E.; Stolarczyk, A.; Drewniak, S.; Urbańczyk, M.; Setkiewicz, M.; Gut, K.; Opilski, Z. Gas sensors based on nanostructures of semiconductors ZnO and TiO2. Bull. Pol. Acad. Sci. Tech. Sci. 2012, 60, 853–859. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Man Ching Ng, A. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526. [Google Scholar] [CrossRef]
- Procek, M.; Pustelny, T.; Stolarczyk, A.; Maciak, E. Studies of changes in electrical resistance of zinc oxide nanostructures under the influence of variable gaseous environments. Bull. Pol. Acad. Sci. Tech. Sci. 2014, 62, 635–639. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Sun, X.; Li, H. Preparation of nanocrystalline metal oxide powders with the surfactant-mediated method. Inorg. Chem. Commun. 2002, 5, 751–755. [Google Scholar] [CrossRef]
- Li, P.; Wei, Y.; Liu, H.; Wang, X. Growth of well-defined ZnO microparticles with additives from aqueous solution. J. Solid State Chem. 2005, 178, 855–860. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.Y.; Leung, Y.H. Recent progress in hydrothermal synthesis of zinc oxide nanomaterials. Recent Pat. Nanotechnol. 2012, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Lou, X. Sol–gel assisted hydrothermal synthesis of ZnO microstructures: Morphology control and photocatalytic activity. Adv. Powder Technol. 2014, 25, 372–378. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.A.; Khusaimi, Z.; Mohammad Rusop, M. A review on zinc oxide nanostructures: Doping and gas sensing. Adv. Mater. Res. 2013, 667, 329–332. [Google Scholar] [CrossRef]
- Procek, M.; Pustelny, T. A study of gas sensing properties of ZnO nanostructures activated by UV light. Photonics Lett. Pol. 2015, 7, 50–52. [Google Scholar]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- An, W.; Wu, X.; Zeng, X.C. Adsorption of O2, H2, CO, NH3 and NO2 on ZnO nanotube: A density functional theory study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Struk, P.; Pustelny, T.; Gołaszewska, K.; Borysiewicz, M.A.; Piotrowska, A. Gas sensors based on ZnO structures. Acta Phys. Pol. A 2013, 124, 567–569. [Google Scholar] [CrossRef]
- Gaman, V.I. Physical principles of operation of oxidizing gas sensors based on metal oxide semiconductors. Russ. Phys. J. 2012, 54, 1364–1371. [Google Scholar] [CrossRef]
- Ristić, M.; Musić, S.; Ivanda, M.; Popović, S. Sol–gel synthesis and characterization of nanocrystalline ZnO powders. J. Alloys Compd. 2005, 397, L1–L4. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B 2013, 177, 178–195. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, W. Synthesis and characterization of flowerlike ZnO nanostructures via an ethylenediamine-meditated solution route. J. Solid State Chem. 2005, 178, 1139–1144. [Google Scholar] [CrossRef]
- Pustelny, T.; Setkiewicz, M.; Drewniak, S.; MacIak, E.; Stolarczyk, A.; Urbańczyk, M.; Procek, M.; Gut, K.; Opilski, Z.; Pasternak, I.; et al. The sensibility of resistance sensor structures with graphene to the action of selected gaseous media. Bull. Pol. Acad. Sci. Tech. Sci. 2013, 61, 293–300. [Google Scholar]
- Zhao, J.; Yang, T.; Liu, Y.; Wang, Z.; Li, X.; Sun, Y.; Du, Y.; Li, Y.; Lu, G. Enhancement of NO2 gas sensing response based on ordered mesoporous Fe-doped In2O3. Sens. Actuators B 2014, 191, 806–812. [Google Scholar] [CrossRef]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.P.; Bedi, R.K. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Yamazoe, N.; Suematsu, K.; Shimanoe, K. Extension of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuators B 2012, 163, 128–135. [Google Scholar] [CrossRef]
- Procek, M.; Pustelny, T. Analysis of the responses of metal-Oxide semiconductor nanostructures to nitrogen dioxide. Acta Phys. Pol. A 2013, 124, 529–533. [Google Scholar] [CrossRef]
- Mikhaylov, R.V.; Lisachenko, A.A.; Shelimov, B.N.; Kazansky, V.B.; Martra, G.; Coluccia, S. FTIR and TPD study of the room temperature interaction of a NO–Oxygen mixture and of NO2 with titanium dioxide. J. Phys. Chem. C 2013, 117, 10345–10352. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Meyer, B. First-principles study of the polar O-terminated ZnO surface in thermodynamic equilibrium with oxygen and hydrogen. Phys. Rev. B 2003, 69, 45416. [Google Scholar] [CrossRef]
- Chougule, M.A.; Sen, S.; Patil, V.B. Fabrication of nanostructured ZnO thin film sensor for NO2 monitoring. Ceram. Int. 2012, 38, 2685–2692. [Google Scholar] [CrossRef]
- Vyas, R.; Sharma, S.; Gupta, P.; Vijay, Y.K.; Prasad, A.K.; Tyagi, A.K.; Sachdev, K.; Sharma, S.K. Enhanced NO2 sensing using ZnO-TiO2 nanocomposite thin films. J. Alloys Compd. 2013, 554, 59–63. [Google Scholar] [CrossRef]
- Şennik, E.; Kerli, S.; Alver, Ü.; Öztürk, Z.Z. Effect of fluorine doping on the NO2-sensing properties of ZnO thin films. Sens. Actuators B 2015, 216, 49–56. [Google Scholar] [CrossRef]
- Hassan, M.; Afify, A.S.; Tulliani, J.M. Synthesis of ZnO nanoparticles onto sepiolite needles and determination of their sensitivity toward humidity, NO2 and H2. J. Mater. Sci. Technol. 2016, 32, 573–582. [Google Scholar] [CrossRef]
- Harale, N.S.; Kamble, A.S.; Tarwal, N.L.; Mulla, I.S.; Rao, V.K.; Kim, J.H.; Patil, P.S. Hydrothermally grown ZnO nanorods arrays for selective NO2 gas sensing: Effect of anion generating agents. Ceram. Int. 2016, 42, 12807–12814. [Google Scholar] [CrossRef]
- Pawar, R.C.; Lee, J.W.; Patil, V.B.; Lee, C.S. Synthesis of multi-dimensional ZnO nanostructures in aqueous medium for the application of gas sensor. Sens. Actuators B 2013, 187, 323–330. [Google Scholar] [CrossRef]
- Calestani, D.; Zha, M.; Mosca, R.; Zappettini, A.; Carotta, M.C.; Di Natale, V.; Zanotti, L. Growth of ZnO tetrapods for nanostructure-based gas sensors. Sens. Actuators B 2010, 144, 472–478. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T.; Maciak, E. A study of a QCM sensor based on TiO2 nanostructures for the detection of NO2 and explosives vapours in air. Sensors 2015, 15, 9563–9581. [Google Scholar] [CrossRef] [PubMed]
















| Material (Structure) | Synthesis Method | NO2 Concentration | Operating Temperature | Response (Carrier Gas) | Reference |
|---|---|---|---|---|---|
| ZnO (mixture of nanotubes and nanoparticles) | Hydrothermal | 1 ppm | 200 °C | 600% (in nitrogen) 232% (in air) | Current paper |
| ZnO (mixture of nanotubes and nanoparticles) | Hydrothermal | 1 ppm | RT (23 °C) + UV excitation | 431% (in nitrogen) 341% (in air) | Current paper |
| ZnO (nanoporous thin film) | Sol-gel | 100 ppm | 200 °C | 37% (in air) | [47] |
| ZnO (nanoflowers) | Hydrothermal | 20 ppm | 200 °C | 9% (in air) 33% (in nitrogen) | [21] |
| ZnO + 2% TiO2 (nanocomposite) | Wet-chemical route | 20 ppm | 250 °C | 58% (-) | [48] |
| ZnO (F doped thin film) | Chemical spraying method | 1 ppm | 200 °C | 90% (in air) | [49] |
| ZnO (nanoparticles on sepiolite matix) | Participation onto sepiolite matrix | 2.5 ppm | 300 °C | 1.1 (Ra/Rg in air) | [50] |
| ZnO (nanorods) | Plate method with hydrothermal deposition | 10 ppm | 200 °C | 4 (Ra/Rg in air) | [51] |
| ZnO (nanoneedles)/ZnO (cacti-like structures) | Chemical route on glass substrate | 200 ppm | 200 °C | 64% (in air)/89% (in air) | [52] |
| ZnO (Nanotetrapods) | Thermal evaporation controlled oxidation | 20 ppm | 300 °C | 2000% (in air) | [53] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procek, M.; Pustelny, T.; Stolarczyk, A. Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials 2016, 6, 227. https://doi.org/10.3390/nano6120227
Procek M, Pustelny T, Stolarczyk A. Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials. 2016; 6(12):227. https://doi.org/10.3390/nano6120227
Chicago/Turabian StyleProcek, Marcin, Tadeusz Pustelny, and Agnieszka Stolarczyk. 2016. "Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method" Nanomaterials 6, no. 12: 227. https://doi.org/10.3390/nano6120227
APA StyleProcek, M., Pustelny, T., & Stolarczyk, A. (2016). Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials, 6(12), 227. https://doi.org/10.3390/nano6120227