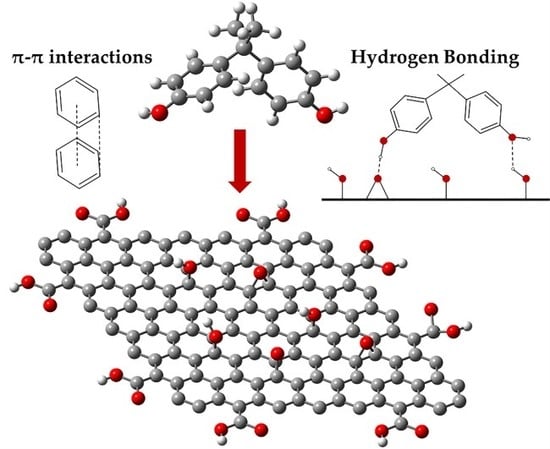
Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution
Abstract
:1. Introduction
2. Results
2.1. Characterization of Synthesized Graphene Oxide
2.2. Parameters for BPA Adsorption on Graphene Oxide
2.2.1. Effect of Contact Time and Initial BPA Concentration
2.2.2. Effect of GO Dosage
2.2.3. Effect of pH
2.2.4. Effect of Temperature and Thermodynamic Study
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Graphene Oxide
4.2.1. Oxidation of Graphite
4.2.2. Washing the Oxidized Graphite
4.3. Characteristic Analysis of the Synthesized Graphene Oxide
4.4. Batch Adsorption Experiments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BPA | Bisphenol A |
| CNMs | Carbon nanomaterials |
| CNTs | Carbon nanotubes |
| DFT | Density functional theory |
| DI | Deionized |
| EDCs | Endocrine disrupting compounds |
| EDS | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| GO | Graphene oxide |
| OCFGs | Oxygen-containing functional groups |
| PTFE | Polytetrafluoroethylene |
| rGO | reduced graphene oxide |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Shen, Z.; Luo, Y.; Wang, Q.; Wang, X.; Sun, R. High-value utilization of lignin to synthesize Ag nanoparticles with detection capacity for Hg2+. ACS Appl. Mater. Interfaces 2014, 6, 16147–16155. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Zhou, X.; Wang, Q.; Shi, X.; Du, Y.; Deng, H.; Jiang, L. Characterization and cytotoxicity study of nanofibrous mats incorporating rectorite and carbon nanotubes. RSC Adv. 2014, 4, 33355–33361. [Google Scholar] [CrossRef]
- Liang, Z.; Zeng, L.; Cao, X.; Wang, Q.; Wang, X.; Sun, R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J. Mater. Chem. C 2014, 2, 9760–9766. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Joseph, L.; Zaib, Q.; Khan, I.A.; Berge, N.D.; Park, Y.-G.; Saleh, N.B.; Yoon, Y. Removal of bisphenol A and 17α-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res. 2011, 45, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Heo, J.; Park, Y.-G.; Flora, J.R.; Yoon, Y. Adsorption of bisphenol A and 17α-ethinyl estradiol on single-walled carbon nanotubes from seawater and brackish water. Desalination 2011, 281, 68–74. [Google Scholar] [CrossRef]
- Latorre, A.; Lacorte, S.; Barceló, D. Presence of nonylphenol, octyphenol and bisphenol A in two aquifers close to agricultural, industrial and urban areas. Chromatographia 2003, 57, 111–116. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Sanhueza, L.; Santander-Nelli, M. Modeling the physisorption of bisphenol A on graphene and graphene oxide. J. Mol. Model. 2013, 19, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, X.; Sun, Y.; Ai, Y.; Wang, X. Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: A combined experimental and theoretical studies. Environ. Sci. Technol. 2015, 49, 9168–9175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Zhu, L.; Tian, X.; Li, S.; Tang, H. Correlation between the adsorption ability and reduction degree of graphene oxide and tuning of adsorption of phenolic compounds. Carbon 2014, 69, 101–112. [Google Scholar] [CrossRef]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhang, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.W.; Bromley, M.; Andrieux, F.P.; Boxall, C. Fabrication and characterisation of the graphene ring micro electrode (GRiME) with an integrated, concentric Ag/AgCl reference electrode. Sensors 2013, 13, 3635–3651. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.R.; Day, S.P.; Woodruff, W.E.; Vallés, C.; Young, R.J.; Kinloch, I.A.; Morley, G.W.; Hanna, J.V.; Wilson, N.R.; Rourke, J.P. Deoxygenation of graphene oxide: Reduction or cleaning? Chem. Mater. 2013, 25, 3580–3588. [Google Scholar] [CrossRef]
- Feng, L.; Gao, G.; Huang, P.; Wang, X.; Zhang, C.; Zhang, J.; Guo, S.; Cui, D. Preparation of Pt Ag alloy nanoisland/graphene hybrid composites and its high stability and catalytic activity in methanol electro-oxidation. Nanoscale Res. Lett. 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, W.; Mao, Y.; Zhang, L.; Cheng, J.; Gong, M.; Zhao, H.; Dai, L.; Zhang, S.; Zhao, Q. Adsorption behavior of copper ions from aqueous solution onto graphene oxide–CdS composite. Chem. Eng. J. 2015, 259, 603–610. [Google Scholar] [CrossRef]
- Li, Z.; Chen, F.; Yuan, L.; Liu, Y.; Zhao, Y.; Chai, Z.; Shi, W. Uranium (VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem. Eng. J. 2012, 210, 539–546. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I.; Karapantsios, T.D.; Matis, K.A. Equilibrium and kinetic modeling of chromium (VI) biosorption by aeromonas caviae. Colloids Surf. A 2004, 242, 93–104. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Mahachai, R.; Chanthai, S. Thermodynamic and kinetic study of the intrinsic adsorption capacity of graphene oxide for malachite green removal from aqueous solution. Orient. J. Chem. 2014, 30, 1463–1474. [Google Scholar] [CrossRef]
- Singanan, M. Biosorption of Hg(II) ions from synthetic wastewater using a novel biocarbon technology. Environ. Eng. Res. 2015, 20, 33–39. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Jacobsen, S.B. The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chem. Geol. Isot. Geosci. Sect. 1987, 66, 245–272. [Google Scholar] [CrossRef]
- AL-Ariqi, W.S.; Ghaleb, A.A. Assessment of hydrochemical quality of ground water under some urban areas within sana’a secreteriat. Eclét. Quím. 2010, 35, 77–84. [Google Scholar] [CrossRef]
- Pearson, P.N.; Palmer, M.R. Middle eocene seawater pH and atmospheric carbon dioxide concentrations. Science 1999, 284, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Byrne, R.H. Spectrophotometric pH measurements of surface seawater at in-situ conditions: Absorbance and protonation behavior of thymol blue. Mar. Chem. 1996, 52, 17–25. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, S. Simultaneous carbon and nitrogen removal from high strength domestic wastewater in an aerobic RBC biofilm. Water Res. 2001, 35, 1714–1722. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A.; Yusoff, M.S. Variability of parameters involved in leachate pollution index and determination of LPI from four landfills in Malaysia. Int. J. Chem. Eng. 2010, 2010. [Google Scholar] [CrossRef]
- Bautista-Toledo, I.; Ferro-Garcia, M.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Fernández, F.V. Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Zhu, C.; Zhao, Y.; Wang, L.; Xie, S.; Wang, Q. Adsorption mechanism of magnetically separable Fe3O4/graphene oxide hybrids. Appl. Surf. Sci. 2015, 355, 562–569. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Chen, N.; Zhou, Y.; Li, B.; Gu, W.; Shi, X.; Xian, Y. Recyclable removal of bisphenol A from aqueous solution by reduced graphene oxide–magnetic nanoparticles: Adsorption and desorption. J. Colloid Interface Sci. 2014, 421, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lashaki, M.J.; Fayaz, M.; Wang, H.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Effect of adsorption and regeneration temperature on irreversible adsorption of organic vapors on beaded activated carbon. Environ. Sci. Technol. 2012, 46, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, M.; Lamp, P.; de la Casa-Lillo, M. Physisorption of hydrogen on microporous carbon and carbon nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, B. Bisphenol A adsorption using reduced graphene oxide prepared by physical and chemical reduction methods. Chem. Eng. Res. Des. 2015, 104, 519–529. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, J.; Liu, K.; Liu, N.; Zhou, B. Adsorption of bisphenol A based on synergy between hydrogen bonding and hydrophobic interaction. Langmuir 2014, 30, 13861–13868. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B.; Xing, B. Wrinkles and folds of activated graphene nanosheets as fast and efficient adsorptive sites for hydrophobic organic contaminants. Environ. Sci. Technol. 2016, 50, 3798–3808. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, S.; Zhao, G.; Wang, Q.; Wang, X. Adsorption of polycyclic aromatic hydrocarbons on graphene oxides and reduced graphene oxides. Chem. Asian J. 2013, 8, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Papageorgiou, A.C.; Lloyd, J.A.; Oh, S.C.; Diller, K.; Allegretti, F.; Klappenberger, F.; Seitsonen, A.P.; Reichert, J.; Barth, J.V. Self-assembly and chemical modifications of bisphenol A on Cu (111): Interplay between ordering and thermally activated stepwise deprotonation. ACS Nano 2013, 8, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Mahvi, A.H.; Rastkari, N.; Saeedi, R.; Nazmara, S.; Iravani, E. Adsorption of bisphenol A (BPA) from aqueous solutions by carbon nanotubes: Kinetic and equilibrium studies. Desalin. Water Treat. 2015, 54, 84–92. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Peng, S.; Fan, X.; Li, S.; Zhang, J. Green synthesis and characterization of graphite oxide by orthogonal experiment. J. Chil. Chem. Soc. 2013, 58, 2213–2217. [Google Scholar] [CrossRef]
- Peng, W.; Liu, S.; Sun, H.; Yao, Y.; Zhi, L.; Wang, S. Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water. J. Mater. Chem. A 2013, 1, 5854–5859. [Google Scholar] [CrossRef]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: an emerging star material for novel separation membranes. J. Mater. Chem. A 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Yang, K.; Chen, B.; Zhu, L. Graphene-coated materials using silica particles as a framework for highly efficient removal of aromatic pollutants in water. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2011, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen bond networks in graphene oxide composite paper: Structure and mechanical properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, B. Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Shen, X.; Zhu, G.; Yuan, A.; Zhang, J.; Ji, Z.; Qiu, D. The influence of wrinkling in reduced graphene oxide on their adsorption and catalytic properties. Carbon 2013, 60, 157–168. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- De Heer, W.A.; Wu, X.; Sprinkle, M.; Berger, C. Method and Apparatus for Producing Graphene Oxide Layers on an Insulating Substrate. U.S. Patent 8173095, 2012. Available online: https://www.google.com/patents/US8173095 (accessed on 02 May 2016). [Google Scholar]
- Krishnan, D.; Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Jang, H.D.; Huang, J. Energetic graphene oxide: Challenges and opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]











| Adsorbent | Weight % | Atomic % | Atomic ratio (C/O) | References | ||||
|---|---|---|---|---|---|---|---|---|
| C | O | Other | C | O | Other | |||
| GO | 49.44 | 40.99 | 9.57 | 58.76 | 36.58 | 4.66 | 1.61 | (this study) |
| 58.36 | 36.85 | 4.79 | 66.45 | 31.50 | 2.05 | 2.11 | [12] | |
| - | - | - | 59.3 | 31.4 | 9.3 | 1.89 | [13] | |
| - | - | - | 58 | 38 | 4 | 1.53 | [14] | |
| - | - | - | 53.32 | 43 | 3.68 | 1.24 | [15] | |
| Temperature (K) | Kd | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol/K) |
|---|---|---|---|---|
| 283 | 3.479 | −3.122 | −18.646 | −54.854 |
| 298 | 2.995 | −2.300 | −18.646 | −54.854 |
| 313 | 1.614 | −1.476 | −18.646 | −54.854 |
| qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| k1 (1/min) | qe,cal (mg/g) | R2 | k2 (g/mg/min) | qe,cal (mg/g) | h (mg/g/min) | R2 | |
| 25.074 | 0.0699 | 3.526 | 0.865 | 0.1042 | 25.126 | 65.789 | 0.9999 |
| 37.379 | 0.1417 | 15.206 | 0.981 | 0.0315 | 37.879 | 45.249 | 0.9998 |
| Temperature (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF | n | R2 | |
| 283 | 62.893 | 0.0534 | 0.9907 | 7.116 | 2.054 | 0.9831 |
| 298 | 49.261 | 0.0627 | 0.9939 | 6.421 | 2.158 | 0.9569 |
| 313 | 32.895 | 0.0495 | 0.9910 | 3.580 | 2.047 | 0.9809 |
| Parameters | Contact Time (min) | Initial BPA Concentration (mg/L) | GO Dosage (mg) | pH | Temperature (K) |
|---|---|---|---|---|---|
| Contact time | 1–60 | 60 | 10 | 3.5 ± 0.5 | 298 |
| Initial BPA concentration | 1–60 | 20, 60 | 10 | 3.5 ± 0.5 | 298 |
| GO dosage | 120 | 60 | 10–200 | 3.5 ± 0.5 | 298 |
| pH | 120 | 60 | 10 | 3–12 | 298 |
| Temperature | 120 | 60 | 10 | 3.5 ± 0.5 | 283–313 |
| Kinetics | 1–60 | 60 | 10 | 3.5 ± 0.5 | 298 |
| Isotherms | 120 | 10–60 | 10 | 3.5 ± 0.5 | 298 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phatthanakittiphong, T.; Seo, G.T. Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution. Nanomaterials 2016, 6, 128. https://doi.org/10.3390/nano6070128
Phatthanakittiphong T, Seo GT. Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution. Nanomaterials. 2016; 6(7):128. https://doi.org/10.3390/nano6070128
Chicago/Turabian StylePhatthanakittiphong, Thatchaphong, and Gyu Tae Seo. 2016. "Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution" Nanomaterials 6, no. 7: 128. https://doi.org/10.3390/nano6070128
APA StylePhatthanakittiphong, T., & Seo, G. T. (2016). Characteristic Evaluation of Graphene Oxide for Bisphenol A Adsorption in Aqueous Solution. Nanomaterials, 6(7), 128. https://doi.org/10.3390/nano6070128