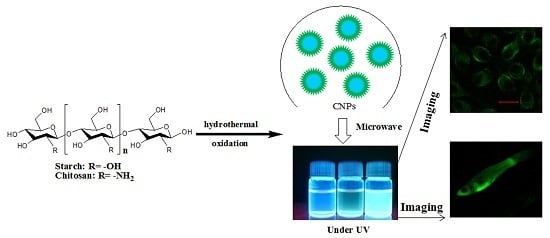
Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging
Abstract
:1. Introduction
2. Results
2.1. Preparation of Polysaccharide-Based NSs
2.2. TEM Characterization of Polysaccharide-Based NSs
2.3. FT-IR Characterization of Polysaccharide-Based NSs
2.4. Fluorescence Properties Analysis of Polysaccharide-Based NSs
2.5. Fluorescence Stability of Polysaccharide-Based NSs
2.6. Effect of Metal Ions on the FL Intensity of Polysaccharide-Based NSs
2.7. pH Stability Experiments of Polysaccharide-Based NSs
2.8. Cytotoxicity Assay
2.9. In Vitro Tumour and Ex Vivo Guppy Fish Imaging
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of Polysaccharide-Based NSs
3.3. Characterization of Polysaccharide-Based NSs
3.4. Photostability Study of Polysaccharide-Based NSs
3.5. Metal Ions Effects on FL Intensity
3.6. pH Effect on FL Intensity of the Polysaccharide-Based NSs
3.7. Cytotoxicity Assay of the Polysaccharide-Based NSs
3.8. In Vitro Mouse Melanoma Cell Imaging
3.9. Ex Vivo Guppy Fish Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NSs | nanostructures |
| QDs | quantum dots |
| UV | ultra-violet |
| FL | fluorescence |
| FWHM | the full width at a half maximum |
| Em | emission wavelength |
| QY | Quantum yield at 360 nm |
| Abs | absorption |
| DMSO | Dimethylsulfoxide |
| TEM | transmission electron microscope |
| FT-IR | Fourier transform infrared |
| MMT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
References
- Alivisatos, A. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.; Chung, L.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.; Bentolila, L.; Tsay, J.; Doose, S.; Li, J.; Sundaresan, G.; Wu, A.; Gambhir, S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Ye, Z.; Wang, G.; Yuan, J. Preparation and time-resolved fluorometric application of luminescent europium nanoparticles. Chem. Mater. 2004, 16, 2494–2498. [Google Scholar] [CrossRef]
- Tan, M.; Wang, G.; Hai, X.; Ye, Z.; Yuan, J. Development of functionalized fluorescent europium nanoparticles for biolabeling and time-resolved fluorometric applications. J. Mater. Chem. 2004, 14, 2896–2901. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.S.; Wang, H.F.; Luo, P.J.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Wang, X.; Wang, H.F.; Lu, F.S.; Luo, P.J.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.F.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.H.; Liu, C.Y.; Ma, D.G. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502–3504. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.L.; Sun, Y.P.; Juzenas, P. Carbon dots as antioxidants and prooxidants. J. Biomed. Nanotechnol. 2011, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 2013, 49, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Lin, L.P.; Wang, X.X.; Jiao, L.; Cui, M.L.; Jiang, S.L.; Cai, W.L.; Zhang, L.H.; Zheng, Z.Y. Zr(H2O)2EDTA modulated luminescent carbon dots as fluorescent probes for fluoride detection. Analyst 2013, 138, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xue, W.; Chen, H.; Lin, J.M. Peroxynitrous-acid-induced chemiluminescence of fluorescent carbon dots for nitrite sensing. Anal. Chem. 2011, 83, 8245–8251. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, H.M.; Duarte, A.J.; Davis, F.; Higson, S.P.; Esteves da Silva, J.C. Layer-by-layer immobilization of carbon dots fluorescent nanomaterials on single optical fiber. Analyt. Chimica. Acta. 2012, 735, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.L.; Zhang, Z.L.; Huang, B.H.; Peng, J.; Zhang, M.; Pang, D.W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, J.H.; Zhang, J.; Zhang, B.L.; Tang, J.L. Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC. Adv. 2012, 2, 8599–8601. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wang, Y.; Gao, Y.; Li, H.; Dai, T.; Liu, Y.; Huo, Q. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Travas-Sejdic, J. Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem. Commun. 2012, 48, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, H.; Long, Y.; Zhang, L.; Gao, M.; Bai, W. Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon 2011, 49, 3134–3140. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Campbell, D.J.; Andrews, M.J.; Stevenson, K.J. New nanotech from an ancient material: Chemistry demonstrations involving carbon-based soot. J. Chem. Edu. 2012, 89, 1280–1287. [Google Scholar] [CrossRef]
- Tan, M.Q.; Zhang, L.X.; Tang, R.; Song, X.J.; Li, Y.M.; Wu, H.; Wang, Y.F.; Lv, G.J.; Liu, W.F.; Ma, X.J. Enhanced photoluminescence and characterization of multicolor carbon dots using plant soot as a carbon source. Talanta 2013, 115, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.J.; Zheng, H.Z.; Long, Y.J.; Du, J.; Hao, J.Y.; Wang, L.L.; Zhou, D.B. Study on the fluorescence characteristics of carbon dots. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2010, 75, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Sheng, Z.H.; Han, H.Y.; Zou, M.Q.; Li, C.X. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.L.; Yan, Q.X.; Yu, K.; Huang, S.M. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012, 22, 16714–16718. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tan, M.; Zhang, D.; Cheng, F.; Hao, W.; Fan, M.; Ma, X.; Wang, J. Development of multicolor carbon nanoparticles for cell imaging. Talanta 2013, 108, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.F.; Li, J.; Wang, E.K. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.; Biju, V.; Yamaoka, Y.; Nagase, T.; Makita, Y.; Shinohara, Y.; Baba, Y.; Ishikawa, M. Synthesis of CdTe quantum dots using a heterogeneous process at low temperature and their optical and structural properties. Appl. Phys. A 2004, 79, 1833–1838. [Google Scholar] [CrossRef]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Edit. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.S.; Zhu, C.Q. Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 2008, 75, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar] [CrossRef] [PubMed]










| Sample | FWHM 1 (nm) | Size Distribution (nm) | Max Em 2 (nm) | Zeta Potential (mV) | QY 3 (Φ, %) |
|---|---|---|---|---|---|
| Starch NSs | 146 | 8–41 | 420 | −18.0 | 11.12 |
| Chitosan NSs | 110 | 62–85 | 445 | +17.5 | 3.16 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, Y.; Bi, J.; Yan, H.; Wang, H.; Song, Y.; Zhu, B.-W.; Tan, M. Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging. Nanomaterials 2016, 6, 130. https://doi.org/10.3390/nano6070130
Zu Y, Bi J, Yan H, Wang H, Song Y, Zhu B-W, Tan M. Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging. Nanomaterials. 2016; 6(7):130. https://doi.org/10.3390/nano6070130
Chicago/Turabian StyleZu, Yinxue, Jingran Bi, Huiping Yan, Haitao Wang, Yukun Song, Bei-Wei Zhu, and Mingqian Tan. 2016. "Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging" Nanomaterials 6, no. 7: 130. https://doi.org/10.3390/nano6070130
APA StyleZu, Y., Bi, J., Yan, H., Wang, H., Song, Y., Zhu, B. -W., & Tan, M. (2016). Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging. Nanomaterials, 6(7), 130. https://doi.org/10.3390/nano6070130