Synthesis of p-Co3O4/n-TiO2 Nanoparticles for Overall Water Splitting under Visible Light Irradiation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. Characterization
2.3. Photocatalysis
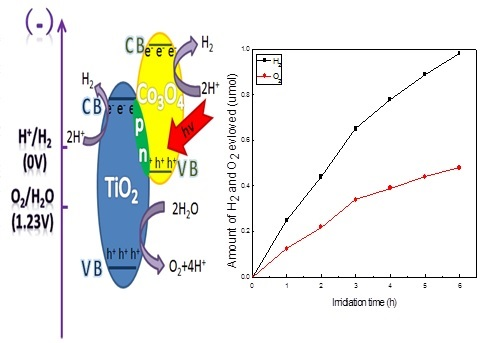
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Yang, P.; Tarascon, J.M. Towards systems materials engineering. Nat. Mater. 2012, 11, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jing, Y.; Li, C.; Zhang, X.F.; Aguinaldo, R.; Alireza, K.; Madsen, K.; Banu, K.; Zhou, Y.C.; Bando, Y.; et al. 3D branched nanowire heterojunction photoelectrodes for high-efficiency solar water splitting and H2 generation. Nanoscale 2012, 4, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Photocatalytic properties of rutile TiO2 powder for overall water splitting. Catal. Sci. Technol. 2014, 4, 1949–1953. [Google Scholar] [CrossRef]
- Guo, S.Y.; Zhao, T.J.; Jin, Z.Q.; Wan, X.M.; Wang, P.G.; Shang, J.; Han, S. Self-assembly synthesis of precious-metal-free 3D ZnO nano/micro spheres with excellent photocatalytic hydrogen production from solar water splitting. J. Power Sources 2015, 293, 17–22. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.H.A.; Kobayashi, Y.; Hernandez-Pagan, E.A.; Hoertz, P.G.; Moore, T.A.; Moore, A.L.; Gust, D.; Mallouk, T.E. Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical cell. J. Am. Chem. Soc. 2009, 131, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Peng, T.; Cai, P.; Yi, H.; Yan, C. Hydrothermal synthesis of flaky crystallized La2Ti2O7 for producing hydrogen from photocatalytic water splitting. Catal. Lett. 2007, 113, 54–58. [Google Scholar] [CrossRef]
- Meng, F.; Cushing, S.K.; Li, J.; Hao, S.; Wu, N. Enhancement of Solar Hydrogen Generation by Synergistic Interaction of La2Ti2O7 Photocatalyst with Plasmonic Gold Nanoparticles and Reduced Graphene Oxide Nanosheets. ACS Catal. 2015, 5, 1949–1955. [Google Scholar] [CrossRef]
- Shi, N.; Cheng, W.; Zhou, H.; Fan, T.; Niederberger, M. Facile synthesis of monodisperse Co3O4 quantum dots with efficient oxygen evolution activity. Chem. Commun. 2015, 51, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, N.; Deng, D.; Li, Y.; Xing, X.; Wang, Y. Meso- and macroporous coral-like Co3O4 for VOCs gas sensor. Ceram. Int. 2015, 41, 11004–11012. [Google Scholar] [CrossRef]
- Ge, B.; Li, K.; Fu, Z.; Pu, L.; Zhang, X. The addition of ortho-hexagon nano spinel Co3O4 to improve the performance of activated carbon air cathode microbial fuel cell. Bioresour. Technol. 2015, 195, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, T.; Jiang, J.; Ai, L. Hierarchically porous Co3O4 architectures with honeycomb-like structures for efficient oxygen generation from electrochemical water splitting. J. Power Sources 2015, 294, 103–111. [Google Scholar] [CrossRef]
- An, H.L.; An, G.H.; Ahn, H.J. Octahedral Co3O4/carbon nanofiber composite-supported Pt catalysts for improved methanol electrooxidation. J. Alloys Compd. 2015, 645, 317–321. [Google Scholar] [CrossRef]
- Soomro, R.A.; Ibupoto, Z.H.; Sherazi, S.T.H.; Abro, M.I.; Willander, M.; Mahesar, S.A.; Kalwar, N.H. Glycine-assisted preparation of Co3O4 nanoflakes with enhanced performance for non-enzymatic glucose sensing. Mater. Express 2015, 5, 437–444. [Google Scholar] [CrossRef]
- Zhang, N.; Shi, J.; Mao, S.S.; Guo, L. Co3O4 quantum dots: Reverse micelle synthesis and visible-light-driven photocatalytic overall water splitting. Chem. Commun. 2014, 50, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, M. Band structure engineering of TiO2 nanowires by n-p codoping for enhanced visible-light photoelectrochemical water-splitting. Phys. Chem. Chem. Phys. 2013, 15, 18523–18529. [Google Scholar] [CrossRef] [PubMed]
- Shaban, Y.A.; Khan, S.U.M. Photoresponse of Visible Light Active CM-n-TiO2, HM-n-TiO2, CM-n-Fe2O3, and CM-p-WO3 towards Water Splitting Reaction. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Odetola, C.; Trevani, T.L.; Easton, E.B. Enhanced activity and stability of Pt/TiO2/carbon fuel cell electrocatalyst prepared using a glucose modifier. J. Power Sources 2015, 294, 254–263. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Diwu, J.; Zhang, Q.; Xu, H.; Chou, X.; Tang, J.; Xue, C. A Green and Facile Synthesis of Carbon-incorporated Co3O4 Nanoparticles and Their Photocatalytic Activity for Hydrogen Evolution. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Liu, J.; Liu, N.; Qiao, S.; Han, Y.; Liu, Y.; Huang, H.; Kang, Z. Carbon quantum dots/SnO2-Co3O4 composite for highly efficient electrochemical water oxidation. Carbon 2015, 92, 64–73. [Google Scholar] [CrossRef]
- Li, H.; Fei, G.T.; Fang, M.; Cui, P.; Guo, X.; Yan, P.; Zhang, L.D. Synthesis of urchin-like Co3O4 hierarchical micro/nanostructures and their photocatalytic activity. Appl. Surf. Sci. 2011, 257, 6527–6530. [Google Scholar] [CrossRef]
- Kwak, G.; Hwang, J.; Cheon, J.Y.; Woo, M.H.; Jun, K.W.; Lee, J.; Ha, K.S. Preparation method of Co3O4 nanoparticles using ordered mesoporous carbons as a template and their application for fischer–tropsch synthesis. J. Phys. Chem. C 2013, 117, 1773–1779. [Google Scholar] [CrossRef]
- Kuo, C.S.; Tseng, Y.H.; Huang, C.H.; Li, Y.Y. Carbon-containing nano-titania prepared by chemical vapor deposition and its visible-light-responsive photocatalytic activity. J. Mol. Catal. A 2007, 270, 93–100. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven NF-codoped TiO2 photocatalysts. Optical characterization, photocatalysis, and potential application to air purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, L.; Wu, X.L.; Yuan, F.; Guo, Y.G.; Ma, Y.; Yao, J. Synthesis of single-crystalline Co3O4 octahedral cages with tunable surface aperture and their lithium storage properties. J. Phys. Chem. C 2009, 113, 15553–15558. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Yang, J.; Guo, L.; Si, Y.; Wang, Y.J.; Yuan, H. Facile carbonaceous microsphere templated synthesis of Co3O4 hollow spheres and their electrochemical performance in supercapacitors. Nano Res. 2013, 6, 87–98. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, G.X.; Bao, S.J.; Guo, J.; Lei, C.; Cai, C.J.; Zeng, J.D.; Wang, R.Y. One step microwave synthesis and magnetic properties of Co3O4 octahedrons. Mater. Lett. 2012, 83, 195–197. [Google Scholar] [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method: 3. Formation process and size control. J. Colloid Interface Sci. 2003, 259, 43–52. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Guo, Y.; Wang, Y.; Guo, Y. Effect of TiO2 crystal structure on the catalytic performance of Co3O4/TiO2 catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 1268–1275. [Google Scholar] [CrossRef]
- Ernsberger, C.; Nickerson, J.; Smith, T.; Miller, A.E.; Banks, D. Low temperature oxidation behavior of reactively sputtered TiN by X-ray photoelectron spectroscopy and contact resistance measurements. J. Vac. Sci. Technol. A 1986, 4, 2784–2788. [Google Scholar] [CrossRef]
- Rubinstein, R.Y.; Shapiro, A. Discrete Event Systems: Sensitivity Analysis and Stochastic Optimization by the Score Function Method; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1993. [Google Scholar]
- Zhang, S.; Li, X.; Chen, J.P. An XPS study for mechanisms of arsenate adsorption onto a magnetite-doped activated carbon fiber. J. Colloid Interface Sci. 2010, 343, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Meng, X.; Wang, T.; Liu, L.; Chang, K.; Liu, G.G.; Ye, J. A highly durable p-LaFeO3/n-Fe2O3 photocell for effective water splitting under visible light. Chem. Commun. 2015, 51, 3630–3633. [Google Scholar] [CrossRef] [PubMed]








| Reference | Materials | Light Source | H2 Amount | O2 Amount | Sacrificial Agent |
|---|---|---|---|---|---|
| [8] | 3D ZnO microspheres | Visible light | 343.75 μmol/h | Methanol, ethanol, formaldehyde | |
| [12] | Co3O4 Quantum Dot | Visible light | 0.79 μmol/h | 0.4 μmol/h | none |
| [7] | Pt-TiO2 | Ultraviolet light | 56.6 μmol/h | 26.5 μmol/h | none |
| [11] | Au@Pt-NLTO/rGO | Visible light | 166.67 μmol/h·g | ethanol | |
| [39] | p-LaFeO3/Fe2O3 | Visible light | 80 μmol/h | 40 μmol/h | none |
| [19] | Co-TiO2 | Visible light | 11 μmol/h·g | 5 μmol/h·g | none |
| [23] | Co3O4 | Visible light | 42.5 μmol/h·g | ethanol | |
| This work | Co3O4 | Visible light | 6.5 μmol/h·g | none | |
| This work | Co3O4-TiO2 | Visible light | 8.16 μmol/h·g | 4.0 μmol/h·g | none |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Hai, Z.; Jian, A.; Xu, H.; Xue, C.; Sang, S. Synthesis of p-Co3O4/n-TiO2 Nanoparticles for Overall Water Splitting under Visible Light Irradiation. Nanomaterials 2016, 6, 138. https://doi.org/10.3390/nano6080138
Zhang Q, Hai Z, Jian A, Xu H, Xue C, Sang S. Synthesis of p-Co3O4/n-TiO2 Nanoparticles for Overall Water Splitting under Visible Light Irradiation. Nanomaterials. 2016; 6(8):138. https://doi.org/10.3390/nano6080138
Chicago/Turabian StyleZhang, Qiang, Zhenyin Hai, Aoqun Jian, Hongyan Xu, Chenyang Xue, and Shengbo Sang. 2016. "Synthesis of p-Co3O4/n-TiO2 Nanoparticles for Overall Water Splitting under Visible Light Irradiation" Nanomaterials 6, no. 8: 138. https://doi.org/10.3390/nano6080138
APA StyleZhang, Q., Hai, Z., Jian, A., Xu, H., Xue, C., & Sang, S. (2016). Synthesis of p-Co3O4/n-TiO2 Nanoparticles for Overall Water Splitting under Visible Light Irradiation. Nanomaterials, 6(8), 138. https://doi.org/10.3390/nano6080138