DNA-Based Enzyme Reactors and Systems
Abstract
:1. Introduction
2. Building with DNA Molecules and Enzymes
2.1. DNA Nanostructures
2.2. DNA-Enzyme Conjugates and Arrays
3. Enzyme Reactors and Cascades
4. Enzymatic Nanodevices with Motion
4.1. Mechanical Regulatory DNA-Enzyme Devices
4.2. Autonomous Molecular Systems
5. Enzyme Containers and Carriers
5.1. DNA Containers for Enzymes
5.2. Cellular Delivery of DNA-Enzyme Conjugates
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| β-gal | β-galactosidase |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| AFM | Atomic force microscopy |
| B-DNA | Double-helical DNA in B-form (geometry attribute) |
| BSA | Bovine serum albumin |
| DNA | Deoxyribonucleic acid |
| dsDNA | double-stranded DNA |
| DX | Double-crossover |
| eGFP | Enhanced green fluorescent protein |
| FRET | Förster resonance energy Transfer |
| G6pDH | Glucose-6-phosphate dehydrogenase |
| GOx | Glucose oxidase |
| HRP | Horseradish peroxidase |
| LDH | Lactate dehydrogenase |
| LUC | Lucia luciferase |
| MDH | Malate dehydrogenase |
| NAD+ | Nicotinamide adenine dinucleotide (oxidized) |
| NADH | Nicotinamide adenine dinucleotide (reduced) |
| nt | nucleotide |
| NTV | NeutrAvidin |
| OAA | Oxaloacetate |
| OAD | Oxaloacetate decarboxylase |
| PDMAEMA | Poly(dimethylaminoethyl methacrylate) |
| PEG | Polyethylene glycol |
| PMS | Phenazine methosulfate |
| RNA | Ribonucleic acid |
| ssDNA | Single-stranded DNA |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| XDR | Xylitol dehydrogenase |
| XR | Xylose reductase |
References
- Vriezema, D.M.; Comellas Aragones, M.; Elemans, J.A.A.W.; Cornelissen, J.L.L.M.; Rowan, A.E.; Nolte, R.J.M. Self-assembled nanoreactors. Chem. Rev. 2005, 105, 1445–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol-gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Van Dongen, S.F.M.; Nallani, M.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; van Hest, J.C.M. A three-enzyme cascade reaction through positional assembly of enzymes in a polymersome nanoreactor. Chem. Eur. J. 2009, 15, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.P.; Prevelige, P.E.; Douglas, T. Nanoreactors by programmed enzyme encapsulation inside the capsid of the bacteriophage P22. ACS Nano 2012, 6, 5000–5009. [Google Scholar] [CrossRef] [PubMed]
- Minten, I.J.; Hendriks, L.J.A.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Controlled encapsulation of multiple proteins in virus capsids. J. Am. Chem. Soc. 2009, 131, 17771–17773. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.P.; Schwarz, B.; Waters, R.S.; Gedeon, T.; Douglas, T. Encapsulation of an enzyme cascade within the bacteriophage P22 virus-like particle. ACS Chem. Biol. 2014, 9, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Brodin, J.D.; Auyeung, E.; Mirkin, C.A. DNA-mediated engineering of multicomponent enzyme crystals. Proc. Natl. Acad. Sci. USA 2015, 112, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Liljeström, V.; Mikkilä, J.; Kostiainen, M.A. Self-assembly and modular functionalization of three-dimensional crystals from oppositely charged proteins. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Linko, V.; Dietz, H. The enabled state of DNA nanotechnology. Curr. Opin. Biotechnol. 2013, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Birktoft, J.J.; Chen, Y.; Wang, T.; Sha, R.; Constantinou, P.E.; Ginell, S.L.; Mao, C.; Seeman, N.C. From molecular to macroscopic via the rational design of a self-assembled 3D DNA crystal. Nature 2009, 461, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.E.; Kilchherr, F.; Kim, D.-N.; Shiao, E.L.; Wauer, T.; Wortmann, P.; Bathe, M.; Dietz, H. A primer to scaffolded DNA origami. Nat. Methods 2011, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Douglas, S.M.; Shih, W.M. Folding DNA into twisted and curved nanoscale shapes. Science 2009, 325, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ong, L.L.; Shih, W.M.; Yin, P. Three-dimensional structures self-assembled from DNA bricks. Science 2012, 338, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.; Mohammed, A.; Gardell, J.; Masich, S.; Czeizler, E.; Orponen, P.; Högberg, B. DNA rendering of polyhedral meshes at the nanoscale. Nature 2015, 523, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, R.; Ratanalert, S.; Zhang, K.; Zhang, F.; Yan, H.; Chiu, W.; Bathe, M. Designer nanoscale DNA assemblies programmed from the top down. Science 2016, 352. [Google Scholar] [CrossRef] [PubMed]
- Gerling, T.; Wagenbauer, K.F.; Neuner, A.M.; Dietz, H. Dynamic DNA devices and assemblies formed by shape-complementary, non-basepairing 3D components. Science 2015, 347, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.-M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Linko, V.; Tapio, K.; Kostiainen, M.A.; Toppari, J.J. Custom-shaped metal nanostructures based on DNA origami silhouettes. Nanoscale 2015, 7, 11267–11272. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, R.; Avendaño, M.S.; Woehrstein, J.B.; Dai, M.; Shih, W.M.; Yin, P. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and exchange-PAINT. Nat. Methods 2014, 11, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Maune, H.T.; Han, S.-P.; Barish, R.D.; Bockrath, M.; Goddard III, W.A.; Rothemund, P.W.K.; Winfree, E. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nat. Nanotechnol. 2010, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Linko, V.; Dietz, H.; Toppari, J.J. Dielectrophoretic trapping of multilayer DNA origami nanostructures and DNA origami-induced local destruction of silicon dioxide. Electrophoresis 2015, 36, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Langecker, M.; Arnaut, V.; Martin, T.G.; List, J.; Renner, S.; Mayer, M.; Dietz, H.; Simmel, F.C. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 2012, 338, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Saccà, B.; Niemeyer, C.M. Functionalization of DNA nanostructures with proteins. Chem. Soc. Rev. 2011, 40, 5910–5921. [Google Scholar] [CrossRef] [PubMed]
- Engelen, W.; Janssen, B.M.G.; Merkx, M. DNA-based control of protein activity. Chem. Commun. 2016, 52, 3587–3722. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, T.E.; Brandl, C.J.; Struhl, K.; Harrison, S.C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: Crystal structure of the protein-DNA complex. Cell 1992, 71, 1223–1237. [Google Scholar] [CrossRef]
- Wilner, O.I.; Weizmann, Y.; Gill, R.; Lioubashevski, O.; Freeman, R.; Willner, I. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotechnol. 2009, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Teller, C.; Willner, I. Organizing protein-DNA hybrids as nanostructures with programmed functionalities. Trends Biotechnol. 2010, 28, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Erkelenz, M.; Kuo, C.-H.; Niemeyer, C.M. DNA-mediated assembly of cytochrome P450 BM3 subdomains. J. Am. Chem. Soc. 2011, 133, 16111–16118. [Google Scholar] [CrossRef] [PubMed]
- Delebeque, C.J.; Lindner, A.B.; Silver, P.A.; Aldaye, F.A. Organization of intracellular reactions with rationally designed RNA assemblies. Science 2011, 333, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Conrado, R.J.; Varner, J.D.; DeLisa, M.P. Engineering the spatial organization of metabolic enzymes: mimicking nature’s synergy. Curr. Opin. Biotechnol. 2008, 19, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Roberts, C.; Toop, A.; Chang, C.E.; Wheeldon, I. Mechanisms of enhanced catalysis in enzyme-DNA nanostructures revealed through molecular simulations and experimental analysis. ChemBioChem 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.P. Substrate channeling and enzyme complexes for biotechnological applications. Biotechnol. Adv. 2011, 29, 715–525. [Google Scholar] [CrossRef] [PubMed]
- Küchler, A.; Yoshimoto, M.; Luginbühl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Simmel, F.C. DNA-based assembly lines and nanofactories. Curr. Opin. Biotechnol. 2012, 23, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, M.; Liu, Y.; Woodbury, N.W.; Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 2012, 134, 5516–5519. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zeng, D.; Chao, J.; Jin, Y.; Zhang, Z.; Liu, H.; Li, D.; Ma, H.; Huang, Q.; Gothelf, K.V.; Fan, C. Single-step rapid assembly of DNA origami nanostructures for addressable nanoscale bioreactors. J. Am. Chem. Soc. 2013, 135, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, Y.R.; Johnson-Buck, A.; Lu, M.; Liu, Y.; Walter, N.G.; Woodbury, N.W.; Yan, H. Multi-enzyme complexes on DNA scaffolds capable of substrate channeling with an artificial swinging arm. Nat. Nanotechnol. 2014, 9, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Linko, V.; Eerikäinen, M.; Kostiainen, M.A. A DNA origami-based enzyme cascade nanoreactor. Chem. Commun. 2015, 51, 5351–5354. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.A.; Nakata, E.; Saimura, M.; Morii, T. Spatially organized enzymes drive cofactor-coupled cascade reactions. J. Am. Chem. Soc. 2016, 138, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fu, J.; Qi, X.; Wootten, S.; Woodbury, N.W.; Liu, Y.; Yan, H. A three-enzyme pathway with an optimized geometric arrangement to facilitate substrate transfer. ChemBioChem 2016, 17, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fu, J.; Hejesen, C.; Yang, Y.; Woodbury, N.W.; Gothelf, K.V.; Liu, Y.; Yan, H. A DNA tweezer-actuated enzyme nanoreactor. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Adendorff, M.R.; Liu, M.; Yan, H.; Bathe, M.; Walter, N.G. Rational design of DNA-actuated enzyme nanoreactors guided by single molecule analysis. Nanoscale 2016, 8, 3125–3137. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhou, C.; Yang, Z.; Liu, D. Regulation of an enzyme cascade reaction by a DNA machine. Small 2013, 9, 3088–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, Z.; Liu, D. Reversible regulation of protein binding affinity by a DNA machine. J. Am. Chem. Soc. 2012, 134, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Wang, M.; Dong, Y.; Zhou, C.; Isbell, M.A.; Yang, Z.; Liu, H.; Liu, D. A switchable DNA origami nanochannel for regulating molecular transport at the nanometer scale. Nanoscale 2016, 8, 3944–3948. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Meyer, T.; Shih, W.M.; Bellot, G. Regulation at a distance of biomolecular interactions using a DNA origami nanoactuator. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhu, Z.; Wu, C.; Peng, L.; Zhou, L.; Gulbakan, B.; Zhu, G.; Williams, K.R.; Tan, W. A logical molecular circuit for programmable and autonomous regulation of protein activity using DNA aptamer-protein interactions. J. Am. Chem. Soc. 2012, 134, 20797–20804. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Dong, M.; Nielsen, M.M.; Jahn, K.; Subramani, R.; Mamdouh, W.; Golas, M.M.; Sander, B.; Stark, H.; Oliveira, C.L.P.; et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 2009, 459, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Sharma, J.; Liu, M.; Jahn, K.; Liu, Y.; Yan, H. Scaffolded DNA origami of a DNA tetrahedron molecular container. Nano Lett. 2009, 9, 2445–2447. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, A.; Komiyama, M. Design and construction of a box-shaped 3D-DNA origami. Chem. Commun. 2009, 28, 4182–4184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fu, J.; Dhakal, S.; Johnson-Buck, A.; Liu, M.; Zhang, T.; Woodbury, N.W.; Liu, Y.; Walter, N.G.; Yan, H. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kiviaho, J.K.; Linko, V.; Ora, A.; Tiainen, T.; Järvihaavisto, E.; Mikkilä, J.; Tenhu, H.; Nonappa, J.; Kostiainen, M.A. Cationic polymers for DNA origami coating—Examining the binding efficiency and tuning the enzymatic reaction rates. Nanoscale 2016, 8, 11674–11680. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.E.; Cha, S.S.; Man, H.-Y.; Han, X. Light-triggered release of bioactive molecules from DNA nanostructures. Nano Lett. 2016, 16, 2781–2785. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.; Iacovelli, F.; Falconi, M.; Kragh, S.L.; Christensen, B.; Frøhlich, R.; Franch, O.; Kristoffersen, E.L.; Stougaard, M.; Leong, K.W.; et al. Temperature-controlled encapsulation and release of an active enzyme in the cavity of a self-assembled DNA nanocage. ACS Nano 2013, 7, 9724–9734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodin, J.D.; Sprangers, A.J.; McMillan, J.R.; Mirkin, C.A. DNA-mediated cellular delivery of functional enzymes. J. Am. Chem. Soc. 2015, 137, 14838–14841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Groves, B.; Muscat, R.A.; Seelig, G. DNA nanotechnology from the test tube to the cell. Nat. Nanotechnol. 2015, 10, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Linko, V.; Ora, A.; Kostiainen, M.A. DNA nanostructures as smart drug-delivery vehicles and molecular devices. Trends Biotechnol. 2015, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Brglez, J.; Nikolov, P.; Angelin, A.; Niemeyer, C.M. Designed intercalators for modification of DNA origami surface properties. Chem. Eur. J. 2015, 21, 9440–9446. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Shih, W.M. Virus-inspired membrane encapsulation of DNA nanostructures to achieve in vivo stability. ACS Nano 2014, 8, 5132–5140. [Google Scholar] [CrossRef] [PubMed]
- Mikkilä, J.; Eskelinen, A.-P.; Niemelä, E.H.; Linko, V.; Frilander, M.J.; Törmä, P.; Kostiainen, M.A. Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett. 2014, 14, 2196–2200. [Google Scholar] [CrossRef] [PubMed]





| Type | Function | Key Aspects |
|---|---|---|
| A glucose oxidase (GOx) – horseradish peroxidase (HRP) cascade on a DNA origami [43]. | The enzyme positions on the DNA origami template can be tuned. | The cascade activity is highly dependent on the spacing between the enzymes; the highest activity was found at a 10 nm distance. |
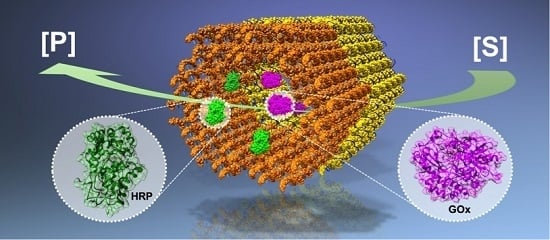
| A GOx-HRP cascade on a DNA origami that can be rolled into tubular shape [44]. | The idea is similar to the above, but here the semi-confined tubular geometry could enable shielding. | The enzymes in the semi-confined geometry show higher enzymatic activity than the free enzyme controls. |
| A swinging arm between malate dehydrogenase (MDH) and glucose-6-phosphate dehydrogenase (G6pDH) assembled on a double-crossover (DX) DNA tile [45]. | The DNA strand acts as a flexible arm that channels the cofactor transfer between the hydrogenases in the complex. | The enzyme activity achieved by the swinging arm is significantly higher than in the case of freely diffusing cofactor. |
| A tubular DNA origami nanoreactor with GOx-HRP pairs [46]. | The nanoreactor is comprised of two units: GOx- and HRP-loaded DNA origamis that can be combined into a complete cascade reactor. | Single origami units and the complete reactor equipped with binding sites show higher activity than the controls without binding sites. |
| A xylose reductase (XR) – xylitol dehydrogenase (XDR) cascade on a DNA origami [47]. | The enzymes are attached to origami via DNA-binding protein adaptors resulting in an artificial enzyme cascade. | The efficiency of the cascade reaction is more dependent on the interenzyme distance than that of the cascade reaction with unimolecular transport between two enzymes. |
| A three-enzyme pathway assembled by a DNA nanostructure [48]. | MDH, oxaloacetate decarboxylase (OAD) and lactate dehydrogenase (LDH) are organized at the corners of the triangular DNA nanostructure, thus forming a three-enzyme cascade. | Activity of the cascade depends more on the geometric patterns of enzymes than the interenzyme spacings. |
| Type | Function | Key Aspects |
|---|---|---|
| DNA nanotweezers [49,50,51,52] equipped with cascade pairs or with the enzyme and its cofactor. | The tweezers can be opened and closed through a strand-displacement reaction. | The enzyme activity can be controlled by switching the tweezers reversibly. |
| A tubular DNA origami nanoreactor [53]. | The lid of the tube can be opened and closed with the help of lock and key strands. | Flowthrough of the compounds into the confined reaction chamber is controlled by the lid. |
| A four-arm DNA origami nanoactuator [54]. | A distance change in a driver site can be propagated to the mirror site containing binding sites for cargo molecules. | The actuator can be driven using different mechanisms, and it can be used for, e.g., tuning fluorescence behavior of enhanced fluorescent protein (eGFP). |
| Aptamer-based logical circuit [55]. | The autonomous logical circuit controls α-thrombin activity through the convertor, controller and generator modules. | α-thrombin aids blood coagulation, and therefore systems such as this may find intriguing biomedical uses. |
| Type | Function | Key Aspects |
|---|---|---|
| Hollow DNA origami containers [56,57,58]. | These structures could be used in encapsulating molecular cargo. | Examples include successful opening and closing mechanisms for conceivable drug release. |
| DNA origami half-cages [59] that can be arranged into a closed box. | The closed geometry shields the enzymatic reactions (such as the glucose oxidase (GOx) – horseradish peroxidase (HRP) cascade) against proteases. | Activity of a single enzyme can be enhanced by encapsulating it into the box. |
| A tubular DNA origami nanocarrier [60]. | The carrier acts as a host for luciferase enzymes. | Luminescence of the cargo can be modulated by coating the carrier with cationic polymers. |
| A box-like DNA origami container [61]. | The box facilitates the binding of proteins (such as bovine serum albumin (BSA)) in the cavity of the origami. | Bound proteins can be released by light. |
| A switchable DNA cage [62]. | The cage can trap and release HRP through a conformational change. | The conformational change can be controlled by temperature. |
| A β-galactosidase (β-gal) protein coated by DNA strands [63]. | DNA-coating significantly increases cellular delivery of the enzymes. | The approach is highly modular, and importantly, the enzymes retain their activity in the transfection. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linko, V.; Nummelin, S.; Aarnos, L.; Tapio, K.; Toppari, J.J.; Kostiainen, M.A. DNA-Based Enzyme Reactors and Systems. Nanomaterials 2016, 6, 139. https://doi.org/10.3390/nano6080139
Linko V, Nummelin S, Aarnos L, Tapio K, Toppari JJ, Kostiainen MA. DNA-Based Enzyme Reactors and Systems. Nanomaterials. 2016; 6(8):139. https://doi.org/10.3390/nano6080139
Chicago/Turabian StyleLinko, Veikko, Sami Nummelin, Laura Aarnos, Kosti Tapio, J. Jussi Toppari, and Mauri A. Kostiainen. 2016. "DNA-Based Enzyme Reactors and Systems" Nanomaterials 6, no. 8: 139. https://doi.org/10.3390/nano6080139
APA StyleLinko, V., Nummelin, S., Aarnos, L., Tapio, K., Toppari, J. J., & Kostiainen, M. A. (2016). DNA-Based Enzyme Reactors and Systems. Nanomaterials, 6(8), 139. https://doi.org/10.3390/nano6080139