Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Ni Nanoparticles
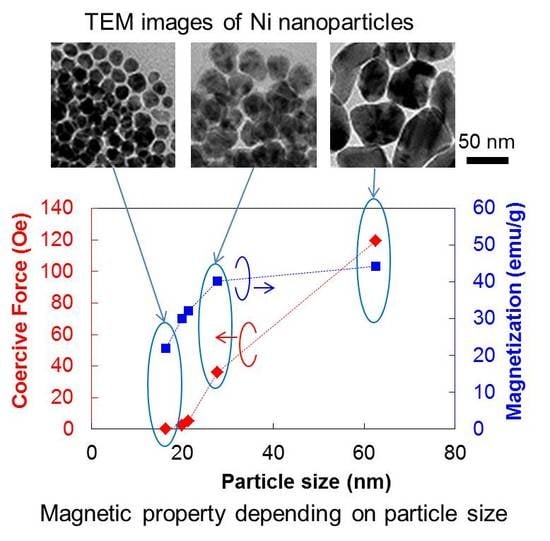
2.2. Magnetic Properties of Ni Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Ni Nanoparticles
4.3. Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willard, M.A.; Kurihara, L.K.; Carpenter, E.E.; Calvin, S.; Harris, V.G. Chemically prepared magnetic nanoparticles. Inter. Mater. Rev. 2004, 49, 125–170. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, C.; Wang, F.; Xil, Z.; Wang, Z.; Deng, Y.; He, N. Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of Magnetic Nanoparticles in Targeted Drug Delivery System. J. Nanosci. Nanotech. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and spin electronics: materials, microstructure and novel properties. J. Mater. Sci. 2006, 41, 793–815. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, I.S. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today 2010, 5, 412–434. [Google Scholar] [CrossRef]
- Bas, J.A.; Calero, J.A.; Dougan, M.J. Sintered soft magnetic materials. Properties and applications. J. Magn. Magn. Mater. 2003, 254, 391–398. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; O’Brien, P. The preparation of organically functionalized chromium and nickel nanoparticles. Chem. Comm. 2001, 19, 1912–1913. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gao, S. Monodisperse nickel nanoparticles prepared from a monosurfactant system and their magnetic properties. J. Mater. Chem. 2003, 13, 1510–1512. [Google Scholar] [CrossRef]
- Chen, D.-H.; Hsieh, C.-H. Synthesis of nickel nanoparticles in aqueous cationic surfactant solutions. J. Mater. Chem. 2002, 12, 2412–2415. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, G.H.; Park, J.; Kim, B.; Chang, Y. Magnetic properties of monodisperse NiHx nanoparticles and comparison to those of monodisperse Ni nanoparticles. J. Phys. Chem. B 2005, 109, 12257–12260. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.T.; Moon, J.Y.; Lee, G.H.; Park, J.; Chang, Y. Comparison of the magnetic properties of metastable hexagonal close-packed Ni nanoparticles with those of the stable face-centered cubic Ni nanoparticles. J. Phys. Chem. B 2006, 110, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhou, H.; Zhang, D.; Wan, H. Synthesis of dodecanethiol monolayer-stabilized nickel nanoparticles. Mater. Sci. Eng. A 2007, 452, 262–266. [Google Scholar] [CrossRef]
- Wu, Z.G.; Munoz, M.; Montero, O. The synthesis of nickel nanoparticles by hydrazine reduction. Adv. Powder Technol. 2010, 21, 165–168. [Google Scholar] [CrossRef]
- Hinotsu, T.; Jeyadevan, B.; Chinnasamy, C.N.; Shinoda, K.; Tohji, K. Size and structure control of magnetic nanoparticles by using a modified polyol process. J. Appl. Phys. 2004, 95, 7477–7479. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Jeyadevan, B.; Shinoda, K.; Tohji, K.; Narayanasamy, A.; Sato, K.; Hisano, S. Synthesis and magnetic properties of face-centered-cubic and hexagonal-close-packed Ni nanoparticles through polyol process. J. Appl. Phys. 2005, 97, 10J309. [Google Scholar] [CrossRef]
- Singh, K.; Kate, K.H.; Chilukuri, V.V.S.; Khanna, P.K. Glycerol mediated low temperature synthesis of nickel nanoparticles by solution reduciton method. J. Nanosci. Nanotechnol. 2011, 11, 5131–5136. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kashiwagi, Y.; Nakamoto, M. Size-controlled synthesis of monodispersed silver nanoparticles capped by long-chain alkyl carboxylates from silver carboxylate and tertiary amine. Langmuir 2006, 22, 8581–8586. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Synthesis and structural and magnetic characterization of Ni(core)/NiO(shell) nanoparticles. ACS Nano 2009, 3, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lee, N.; Park, J.; Kim, B.H.; Yi, Y.-W.; Kim, T.; Kim, T.K.; Lee, I.H.; Paik, S.R.; Hyeon, T. Ni/NiO core/shell nanoparticles for selective binding and magnetic separation of histidine-tagged proteins. J. Am. Chem. Soc. 2006, 128, 10658–10659. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, X.; She, H.; Yue, G.-H.; Peng, D.-L. Size- and structure-controlled synthesis and characterization of nickel nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Y.; Yue, G.-H.; Peng, D.-L.; Luo, X. Preparation of hexagonal close-packed nickel nanoparticles via a thermal decomposition approach using nickel acetate tetrahydrate as a precursor. J. Alloy Compd. 2009, 476, 864–868. [Google Scholar] [CrossRef]
- Carenco, S.; Boissière, C.; Nicole, L.; Sanchez, C.; Floch, P.L.; Mézailles, N. Controlled design of size-tunable monodisperse nickel nanoparticles. Chem. Mater. 2010, 22, 1340–1349. [Google Scholar] [CrossRef]
- Pan, Y.; Jia, R.; Zhao, J.; Liang, J.; Liu, Y.; Liu, C. Size-controlled synthesis of monodisperse nickel nanoparticlesand investigation of their magnetic and catalytic properties. Appl. Surf. Sci. 2014, 316, 276–285. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, D.-L.; Lin, D.; Luo, X. Preparation and magnetic properties of nickel nanoparticles via the thermal decomposition of nickel organometallic precursor in alkylamines. Nanotechnology 2007, 18, 97–102. [Google Scholar] [CrossRef]
- He, X.; Zhong, W.; Au, C.-T.; Du, Y. Size dependence of the magnetic properties of Ni nanoparticles prepared by thermal decomposition method. Nanoscale Res. Lett. 2013, 8, 13315–13321. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Simeonidis, K.; Vilalta-Clemente, A.; Tuna, F.; Tsiaoussis, I.; Angelakeris, M.; Dendrinou-Samara, C.; Kalogirou, O. Controlling the crystal structure of Ni nanoparticles by the use of alkylamines. J. Magn. Magn. Mater. 2009, 321, 2723–2728. [Google Scholar] [CrossRef]
- Sidhaye, D.S.; Bala, T.; Srinath, S.; Srikanth, H.; Poddar, P.; Sastry, M.; Prasad, B.L.V. Preparation of nearly monodisperse nickel nanoparticles by a facile solution based methodology and their ordered assemblies. J. Phys. Chem. C 2009, 113, 3426–3429. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ingham, B.; Cheong, S.; Williams, G.V.; Dotzler, C.; Toney, M.F.; Jefferson, D.A.; Corbos, E.C.; Bishop, P.T.; Cookson, J.; et al. Synthesis, alignment, and magnetic properties of monodisperse nickel nanocubes. J. Am. Chem. Soc. 2012, 134, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi-Montes, N.; Gorria, P.; Martínez-Blanco, D.; Amghouz, Z.; Fuertes, A.B.; Barquín, L.F.; de Pedro, I.; Olivi, L.; Blanco, J.A. Unravelling the onset of the exchange bias effect in Ni(core)@NiO(shell) nanoparticles embedded in a mesoporous carbon matrix. J. Mater. Chem. C 2015, 3, 5674–5682. [Google Scholar] [CrossRef]
- Rinaldi-Montes, N.; Gorria, P.; Martínez-Blanco, D.; Fuertes, A.B.; Barquín, L.F.; Puente-Orench, I.; Blanco, J.A. Bridging exchange bias effect in NiO and Ni(core)@NiO(shell) nanoparticles. J. Magn. Magn. Mater. 2016, 400, 236–241. [Google Scholar] [CrossRef]
- Park, J.; Koo, B.; Yoon, K.Y.; Hwang, Y.; Kang, M.; Park, J.-G.; Hyeon, T. Generalized synthesis of metal phosphide nanorods via thermal decomposition of continuously delivered metal-phosphine complexes using a syringe pump. J. Am. Chem. Soc. 2005, 127, 8433–8440. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, K.; Burns, A.W.; Bussell, M.E.; Brock, S.L. Synthesis and characterization of discrete nickel phosphide nanoparticles: effect of surface ligation chemistry on catalytic hydrodesulfurization of thiophene. Adv. Funct. Mater. 2007, 17, 3933–3939. [Google Scholar] [CrossRef]












© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishizaki, T.; Yatsugi, K.; Akedo, K. Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials 2016, 6, 172. https://doi.org/10.3390/nano6090172
Ishizaki T, Yatsugi K, Akedo K. Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials. 2016; 6(9):172. https://doi.org/10.3390/nano6090172
Chicago/Turabian StyleIshizaki, Toshitaka, Kenichi Yatsugi, and Kunio Akedo. 2016. "Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent" Nanomaterials 6, no. 9: 172. https://doi.org/10.3390/nano6090172
APA StyleIshizaki, T., Yatsugi, K., & Akedo, K. (2016). Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials, 6(9), 172. https://doi.org/10.3390/nano6090172