Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction
Abstract
:1. Introduction
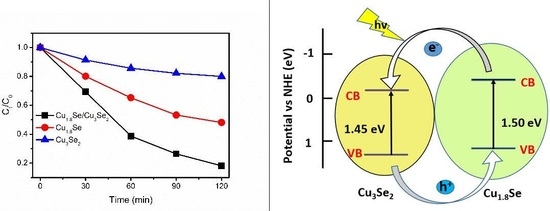
2. Results and Discussion
3. Materials and Methods
3.1. Preparation
3.2. Characterization
3.3. Photocatalytic Test
3.4. Photoelectrochemical Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, X.; Yin, K.; Dong, S.; Ren, Z.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.R.; Pudukudy, M.; Yaakob, Z.; Ismail, N.A. CeO2-TiO2 as a visible light active catalyst for the photoreduction of CO2 to methanol. J. Rare Earths 2015, 33, 1155–1161. [Google Scholar] [CrossRef]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/gC3N4 photocatalyst. Appl. Catal. B 2015, 168, 1–8. [Google Scholar]
- Hou, Y.-F.; Liu, S.-J.; Zhang, J.; Cheng, X.; Wang, Y. Facile hydrothermal synthesis of TiO2–Bi2WO6 hollow superstructures with excellent photocatalysis and recycle properties. Dalton Trans. 2014, 43, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, H.; Fan, Y.; Gu, X.; Zhan, J. One-dimensional CdS/α-Fe2O3 and CdS/Fe3O4 heterostructures: Epitaxial and nonepitaxial growth and photocatalytic activity. J. Phys. Chem. C 2009, 113, 14119–14125. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Ma, X.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.H. Cu2(OH)PO4, a Near-Infrared-Activated Photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 4810–4813. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to Near-Infrared, WS2 Nanosheet: A Novel Photocatalyst for Full Solar Light Spectrum Photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.; Zhao, Q.; Quan, X.; Chen, G. Fabrication of Cu2O/TiO2 nanotube heterojunction arrays and investigation of its photoelectrochemical behavior. Appl. Phys. Lett. 2009, 95, 093108. [Google Scholar] [CrossRef]
- Georgieva, J.; Armyanov, S.; Valova, E.; Poulios, I.; Sotiropoulos, S. Enhanced photocatalytic activity of electrosynthesised tungsten trioxide–titanium dioxide bi-layer coatings under ultraviolet and visible light illumination. Electrochem. Commun. 2007, 9, 365–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Wang, L.; Sun, S. Enhancement of Visible-Light Photocatalysis by Coupling with Narrow-Band-Gap Semiconductor: A Case Study on Bi2S3/Bi2WO6. ACS Appl. Mater. Interfaces 2012, 4, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.; Woo, J.Y.; Lee, K.; Kim, W.D.; Yoo, Y.; Lee, D.C. Extending the Limit of Low-Energy Photocatalysis: Dye Reduction with PbSe/CdSe/CdS Core/Shell/Shell Nanocrystals of Varying Morphologies under Infrared Irradiation. J. Phys. Chem. C 2012, 116, 25407–25414. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-based hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Stevels, A.; Jellinek, F. Phase transitions in copper chalcogenides: I. The copper-selenium system. Recl. Trav. Chim. Pays-Bas. 1971, 90, 273–283. [Google Scholar] [CrossRef]
- Haram, S.K.; Santhanam, K.; Neumann-Spallart, M.; Levy-Clement, C. Electroless deposition on copper substrates and characterization of thin films of copper (I) selenide. Mater. Res. Bull. 1992, 27, 1185–1191. [Google Scholar] [CrossRef]
- Shafizade, R.; Ivanova, I.; Kazinets, M. Electron diffraction study of phase transformations of the compound CuSe. Thin Solid Films 1978, 55, 211–220. [Google Scholar] [CrossRef]
- Garcıa, V.; Nair, P.; Nair, M. Copper selenide thin films by chemical bath deposition. J. Cryst. Growth 1999, 203, 113–124. [Google Scholar] [CrossRef]
- Lakshmi, M.; Bindu, K.; Bini, S.; Vijayakumar, K.; Kartha, C.S.; Abe, T.; Kashiwaba, Y. Chemical bath deposition of different phases of copper selenide thin films by controlling bath parameters. Thin Solid Films 2000, 370, 89–95. [Google Scholar] [CrossRef]
- Lakshmi, M.; Bindu, K.; Bini, S.; Vijayakumar, K.; Kartha, C.S.; Abe, T.; Kashiwaba, Y. Reversible Cu2−xSe↔Cu3Se2 phase transformation in copper selenide thin films prepared by chemical bath deposition. Thin Solid Films 2001, 386, 127–132. [Google Scholar] [CrossRef]
- Heyding, R.D.; Murray, R.M. The crystal structures of Cu1.8Se, Cu3Se2, α-and γCuSe, CuSe2, and CuSe2II. Can. J. Chem. 1976, 54, 841–848. [Google Scholar] [CrossRef]
- Heyding, R. The copper/selenium system. Can. J. Chem. 1966, 44, 1233–1236. [Google Scholar] [CrossRef]
- Bhuse, V.; Hankare, P.; Garadkar, K.; Khomane, A. A simple, convenient, low temperature route to grow polycrystalline copper selenide thin films. Mater. Chem. Phys. 2003, 80, 82–88. [Google Scholar] [CrossRef]
- Lévy-Clément, C.; Neumann-Spallart, M.; Haram, S.; Santhanam, K. Chemical bath deposition of cubic copper (I) selenide and its room temperature transformation to the orthorhombic phase. Thin Solid Films 1997, 302, 12–16. [Google Scholar] [CrossRef]
- Chen, W.S.; Stewart, J.; Mickelsen, R. Polycrystalline thin-film Cu2−xSe/CdS solar cell. Appl. Phys. Lett. 1985, 46, 1095–1097. [Google Scholar] [CrossRef]
- Dorfs, D.; Härtling, T.; Miszta, K.; Bigall, N.C.; Kim, M.R.; Genovese, A.; Falqui, A.; Povia, M.; Manna, L. Reversible Tunability of the Near-Infrared Valence Band Plasmon Resonance in Cu2–xSe Nanocrystals. J. Am. Chem. Soc. 2011, 133, 11175–11180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Zhou, B.; Law, W.C.; Cartwright, A.N.; Swihart, M.T. Size-Controlled Synthesis of Cu2−xE (E = S, Se) Nanocrystals with Strong Tunable Near-Infrared Localized Surface Plasmon Resonance and High Conductivity in Thin Films. Adv. Funct. Mater. 2013, 23, 1256–1264. [Google Scholar] [CrossRef]
- Zhu, J.; Palchik, O.; Chen, S.; Gedanken, A. Microwave assisted preparation of CdSe, PbSe, and Cu2−xSe nanoparticles. J. Phys. Chem. B 2000, 104, 7344–7347. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, X.; Jiang, X.; Lu, J.; Zhu, L. Sonochemical Synthesis and Mechanistic Study of Copper Selenides Cu2−xSe, β-CuSe, and Cu3Se2. Inorg. Chem. 2002, 41, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, H.; Zhu, J.-J.; Chen, H.-Y. Sonochemical synthesis of copper selenides nanocrystals with different phases. J. Cryst. Growth 2002, 234, 263–266. [Google Scholar] [CrossRef]
- Kemmler, M.; Lazell, M.; O'brien, P.; Otway, D.; Park, J.-H.; Walsh, J. The growth of thin films of copper chalcogenide films by MOCVD and AACVD using novel single-molecule precursors. J. Mater. Sci. Mater. Electron. 2002, 13, 531–535. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhang, M.; Liu, Y.; Zhu, Y. A superior photocatalytic performance of a novel Bi2SiO5 flower-like microsphere via a phase junction. Nanoscale 2014, 6, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Butler, M. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, W.; Chen, C.; Zhao, J.; Shuai, Z. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2−xBx under visible irradiation. J. Am. Chem. Soc. 2004, 126, 4782–4783. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Y.; Huang, B.; Li, H.; Qin, X.; Zhang, X.; Dai, Y. Preparation, characterization, and photocatalytic properties of silver carbonate. Appl. Surf. Sci. 2011, 257, 8732–8736. [Google Scholar] [CrossRef]
- Sakkas, V.; Arabatzis, I.; Konstantinou, I.; Dimou, A.; Albanis, T.; Falaras, P. Metolachlor photocatalytic degradation using TiO2 photocatalysts. Appl. Catal. B 2004, 49, 195–205. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible light photocatalytic H2-production activity of CuS/ZnS porous nanosheets based on photoinduced interfacial charge transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Chiam, S.; Huang, J.; Wang, S.; Pan, J.; Chim, W. Growth of Cu2O on Ga-doped ZnO and their interface energy alignment for thin film solar cells. J. Appl. Phys. 2010, 108, 033702. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Wu, Z.; Jiang, D.; Chen, M. Ion-exchange synthesis of Ag/Ag2S/Ag3CuS2 ternary hollow microspheres with efficient visible-light photocatalytic activity. Dalton Trans. 2014, 43, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.-T.; Lim, T.-T.; Yan, X. Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci. Rep. 2015, 5, 16369. [Google Scholar] [CrossRef] [PubMed]
- Riha, S.C.; Johnson, D.C.; Prieto, A.L. Cu2Se nanoparticles with tunable electronic properties due to a controlled solid-state phase transition driven by copper oxidation and cationic conduction. J. Am. Chem. Soc. 2010, 133, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; An, L.; Pattengale, B.; Kong, Q.; Zhang, X.; Xi, P.; Huang, J. Ultrafast hole trapping and relaxation dynamics in p-type CuS nanodisks. J. Phys. Chem. Lett. 2015, 6, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Savariraj, A.D.; Viswanathan, K.K.; Prabakar, K. Influence of Cu vacancy on knit coir mat structured CuS as counter electrode for quantum dot sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 19702–19709. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Kako, T.; Jinhua, Y.; Zhigang, Z. Band structure design and photocatalytic activity of In2O3/N-InNbO4 composite. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]








| Samples | Cu1.8Se/Cu3Se2 Composite | Cu1.8Se | Cu3Se2 |
|---|---|---|---|
| Size (nm) | 66.3 | 48.6 | 57.8 |
| Specific surface area (m2/g) | 5.842 | 8.655 | 6.556 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.-N.; Wang, H.-C.; Shen, Y.; Lin, Y.-H.; Nan, C.-W. Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction. Nanomaterials 2017, 7, 19. https://doi.org/10.3390/nano7010019
Qiao L-N, Wang H-C, Shen Y, Lin Y-H, Nan C-W. Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction. Nanomaterials. 2017; 7(1):19. https://doi.org/10.3390/nano7010019
Chicago/Turabian StyleQiao, Li-Na, Huan-Chun Wang, Yang Shen, Yuan-Hua Lin, and Ce-Wen Nan. 2017. "Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction" Nanomaterials 7, no. 1: 19. https://doi.org/10.3390/nano7010019
APA StyleQiao, L. -N., Wang, H. -C., Shen, Y., Lin, Y. -H., & Nan, C. -W. (2017). Enhanced Photocatalytic Performance under Visible and Near-Infrared Irradiation of Cu1.8Se/Cu3Se2 Composite via a Phase Junction. Nanomaterials, 7(1), 19. https://doi.org/10.3390/nano7010019