Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Characterization
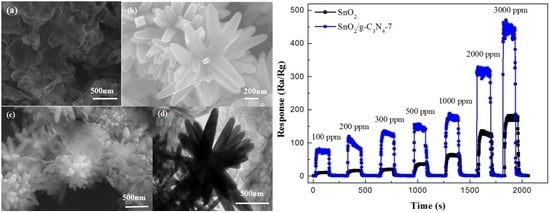
2.2. Sensing Performance Tests
2.3. Mechanism Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. Characterizations
3.4. Gas Sensor Fabrication and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, Y.H.; Ma, D.Q.; Wang, W.J.; Zhang, L.F.; Xu, X.H. A supramolecular self-assembly hydrogel binder enables enhanced cycling of SnO2-based anode for high-performance lithium-ion batteries. J. Mater. Sci. 2017, 52, 3545–3555. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibiyangjeh, A. A simple large-scale method for preparation of g-C3N4/SnO2 nanocomposite as visible-light-driven photocatalyst for degradation of an organic pollutant. Mater. Express 2015, 5, 309–318. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.H.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Wu, M.Y.; Zeng, W.; Li, Y.Q. Hydrothermal synthesis of novel SnO2 nanoflowers and their gas-sensing properties. Mater. Lett. 2013, 104, 34–36. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, Q.P.; Qin, G.H.; Zhao, K.; Du, G.F.; Wang, H.; Zhao, H.Y. Gas sensing characteristics of novel twin-layered SnO2 nanoarray fabricated by substrate-free hydrothermal route. Sens. Actuators B 2015, 218, 205–214. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.J.; Wang, X.X.; Zeng, D.W.; Xie, C.S. Enhancing room-temperature NO2 sensing properties via forming heterojunction for NiO-rGO composited with SnO2 nanoplates. Sens. Actuators B 2016, 243, 1010–1019. [Google Scholar] [CrossRef]
- Choi, S.W.; Katoch, A.; Kim, J.H.; Kim, S.S. Striking sensing improvement of n-type oxide nanowires by electronic sensitization based on work function difference. J. Mater. Chem. C 2015, 3, 1521–1527. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, W.; Hao, J.H.; Li, Y.Q.; Miao, B. Hydrothermal synthesis of flower-like SnO2 architectures with superior gas sensing properties. Mater. Lett. 2015, 145, 133–136. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Liu, J.J.; Chang, H.Y.; Liu, A.M.; Xia, B.K. Characterization of a hybrid composite of SnO2 nanocrystal-decorated reduced graphene oxide for ppm-level ethanol gas sensing application. RSC Adv. 2015, 5, 18666–18672. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sens. Actuators B 2017, 244, 466–474. [Google Scholar] [CrossRef]
- Feng, Q.X.; Li, X.G.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuators B 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Song, Z.L.; Wei, Z.R.; Wang, B.C.; Luo, Z.; Xu, S.M.; Zhang, W.K.; Yu, H.X.; Li, M.; Huang, Z.; Zang, J.F.; et al. Sensitive room-temperature H2S gas sensors employing SnO2 quantum wire/reduced graphene oxide nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Mao, S.; Cui, S.M.; Lu, G.H.; Yu, K.H.; Wen, Z.H.; Chen, J.H. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009–11013. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.C.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, T.S.; Wang, B.Q.; Sun, P.; Yang, Q.Y.; Liang, X.S.; Song, H.W.; Lu, G.Y. Highly sensitive and low detection limit of ethanol gas sensor based on hollow ZnO/SnO2 spheres composite material. Sens. Actuators B 2017, 245, 551–559. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Manikandan, E.; Ramana Reddy, M.V. Enhancing the Isopropanol gas sensing performance of SnO2/ZnO core/shell nanocomposite gas sensor. J. Mater. Chem. C 2017, 5, 2662–2668. [Google Scholar] [CrossRef]
- Silva, L.F.D.; M’Peko, J.-C.; Catto, A.C.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Ribriro, C.; Longo, E. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature. Sens. Actuators B 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, J.T.; Wang, Y.H.; Yu, K.F.; Tang, X.Y.; Zhang, Y.Y.; Wang, S.P.; Wei, C.S. Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 2016, 6, 35079. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Park, S.; Chang, S.P. Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles. Sens. Actuators B 2017, 238, 871–879. [Google Scholar] [CrossRef]
- Wang, T.S.; Huang, Z.S.; Yu, Z.D.; Wang, B.Q.; Wang, H.; Sun, P.; Suo, H.; Gao, Y.; Sun, Y.F.; Li, T.; et al. Low operating temperature toluene sensor based on novel α-Fe2O3/SnO2 heterostructure nanowire arrays. RSC Adv. 2016, 6, 52604–52610. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Liu, J.G.; Zhou, X.; Li, X.W.; Hu, X.L.; Lu, G.Y. Hierarchical assembly of α-Fe2O3 nanosheets on SnO2 hollow nanospheres with enhanced ethanol sensing properties. ACS Appl. Mater. Interface 2015, 7, 19119–19125. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.X.; Zhu, J.H.; Xu, Z.Y.; Huang, X.T. Facile synthesis of α-Fe2O3@SnO2 core–shell heterostructure nanotubes for high performance gas sensors. Sens. Actuators B 2015, 213, 27–34. [Google Scholar] [CrossRef]
- Gu, C.P.; Cui, Y.W.; Wang, L.Y.; Sheng, E.H.; Shim, J.J.; Huang, J.R. Synthesis of the porous NiO/SnO2 microspheres and microcubes and their enhanced formaldehyde gas sensing performance. Sens. Actuators B 2017, 241, 298–307. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Sun, X.H. Electrospun Nanowebs of NiO/SnO2 p-n Heterojunctions for Enhanced Gas Sensing. Appl. Surf. Sci. 2016, 389, 514–520. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.W.; Zhu, Q.; Wu, J.J.; Huang, Q.W.; Zhang, W.; Xie, C.S. Enhanced room temperature NO2 response of NiO-SnO2 nanocomposites induced by interface bonds at the p-n heterojunction. Phys. Chem. Chem. Phys. 2016, 18, 5386–5396. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.P.; Li, L.P.; Li, X.G.; Lin, R.; Li, G.S. Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.H.; Yang, S.L.; Wu, H.S.; Wu, Y.X.; Wu, L.D.; Pan, J.; Xiong, X. In situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, D.K.; Guo, W.M.; Chen, S.J. The α-Fe2O3/g-C3N4 heterostructural nanocomposites with enhanced ethanol gas sensing performance. J. Alloys Compd. 2016, 685, 84–90. [Google Scholar] [CrossRef]
- Zeng, B.R.; Zhang, L.C.; Wan, X.Y.; Song, H.J.; Lv, Y. Fabrication of α-Fe2O3/g-C3N4 composites for cataluminescence sensing of H2S. Sens. Actuators B 2015, 211, 370–376. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Zhang, L.C.; Lv, Y. Dielectric barrier discharge plasma-assisted fabrication of g-C3N4-Mn3O4 composite for high-performance cataluminescence H2S gas sensor. Sens. Actuators B 2017, 239, 1177–1184. [Google Scholar] [CrossRef]
- Mathur, S.; Barth, S.; Shen, H.; Pyun, J.-C.; Werner, U. Size-Dependent Photoconductance in SnO2 Nanowires. Small 2005, 1, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Lao, C.S.; Wang, Z.L.; Xie, Z.X.; Zheng, L.S. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed]
- Paulowicz, I.; Hrkac, V.; Kaps, S.; Cretu, V.; Lupan, O.; Braniste, T.; Duppel, V.; Tiginyanu, L.; Kienle, L.; Adelung, R.; et al. Nanowire Networks: Three-Dimensional SnO2 Nanowire Networks for Multifunctional Applications: From High-Temperature Stretchable Ceramics to Ultraresponsive Sensors. Adv. Electron. Mater. 2015, 1, 1500081. [Google Scholar] [CrossRef]
- Lupan, O.; Braniste, T.; Deng, M.; Ghimpu, L.; Paulowicz, I.; Mishra, Y.K.; Kienle, L.; Adelung, R.; Tiginyanu, I. Rapid switching and ultra-responsive nanosensors based on individual shell–core Ga2O3/GaN:Ox@SnO2 nanobelt with nanocrystalline shell in mixed phases. Sens. Actuators B 2015, 221, 544–555. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.P.; Xu, G.F.; Peng, Y.R.; Gong, L.S.; Li, X.H.; Li, Y.L.; Lin, Y.Y.; Chen, G.N. Highly photoactive heterojunction based on g-C3N4 nanosheets decorated with dendritic zinc(II) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of choline. RSC Adv. 2014, 4, 58226–58230. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Q.Y.; Wang, Z.Y.; Zhang, R.; Gao, Y.; Sun, P.; Sun, Y.F.; Lu, G.Y. Improvement of NO2 gas sensing performance based on discoid tin oxide modified by reduced graphene oxide. Sens. Actuators B 2016, 227, 419–426. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.B.; Ju, D.X.; Cao, B.Q. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuators B 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Chai, G.; Heinrich, H.; Park, S.; Schulte, A. Synthesis of one-dimensional SnO2 nanorods via a hydrothermal technique. Physica E 2009, 41, 533–536. [Google Scholar] [CrossRef]
- Vuong, D.D.; Hien, V.X.; Trung, K.Q.; Chien, N.D. Synthesis of SnO2 micro-spheres, nano-rods and nano-flowers via simple hydrothermal route. Physica E 2011, 44, 345–349. [Google Scholar] [CrossRef]
- Cheng, B.; Russell, J.M.; Shi, W.S.; Zhang, L.; Samulski, E.T. Large-Scale, Solution-Phase Growth of Single-Crystalline SnO2 Nanorods. J. Am. Chem. Soc. 2004, 126, 5972–5973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Huang, J.; Yang, J.D.; Wang, S.R. Pt nanoparticles functionalized 3D SnO2 nanoflowers for gas sensor application. Solid State Electron. 2017, 130, 20–27. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Wu, Q.S. A highly sensitive room-temperature sensing material for NH3: SnO2-nanorods coupled by rGO. Sens. Actuators B 2017, 242, 1216–1226. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhu, L.P.; Wen, Z.; Ye, Z.Z. Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuators B 2017, 238, 1052–1059. [Google Scholar] [CrossRef]
- Cao, J.L.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.X.; Zhang, H.L.; Sun, G.; Hari, B.; Zhang, Z.Y. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]











| Materials | Ethanol Vapor Concentration (ppm) | Temperature (°C) | Response (Ra/Rg) | Ref. |
|---|---|---|---|---|
| RGO/hollow SnO2 | 100 | 300 | 70.4 | [11] |
| Hollow ZnO/SnO2 spheres | 100 | 225 | 78.2 | [16] |
| α-Fe2O3/g-C3N4 | 100 | 340 | 7.76 | [29] |
| Au/3D SnO2 microstructure | 150 | 340 | 30 | [38] |
| SnO2/g-C3N4 | 100 | 340 | 85 | this work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, J.; Qin, C.; Zhang, B.; Sun, G.; Zhang, Z. Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite. Nanomaterials 2017, 7, 285. https://doi.org/10.3390/nano7100285
Wang Y, Cao J, Qin C, Zhang B, Sun G, Zhang Z. Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite. Nanomaterials. 2017; 7(10):285. https://doi.org/10.3390/nano7100285
Chicago/Turabian StyleWang, Yan, Jianliang Cao, Cong Qin, Bo Zhang, Guang Sun, and Zhanying Zhang. 2017. "Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite" Nanomaterials 7, no. 10: 285. https://doi.org/10.3390/nano7100285
APA StyleWang, Y., Cao, J., Qin, C., Zhang, B., Sun, G., & Zhang, Z. (2017). Synthesis and Enhanced Ethanol Gas Sensing Properties of the g-C3N4 Nanosheets-Decorated Tin Oxide Flower-Like Nanorods Composite. Nanomaterials, 7(10), 285. https://doi.org/10.3390/nano7100285