Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
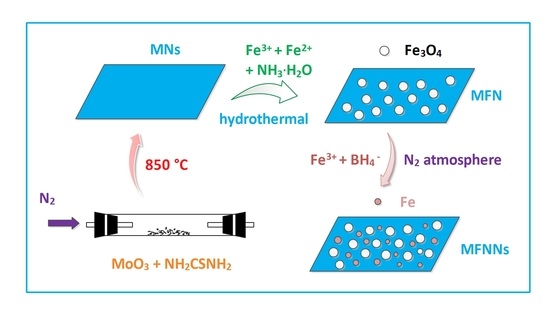
2.2. Synthesis of MoS2 Nanosheets (MNs)
2.3. Synthesis of MoS2/Fe3O4 Nanocomposite (MFN)
2.4. Synthesis of MoS2/Fe3O4/nZVI Nanocomposites (MFNNs)
2.5. Characterization
2.6. Simultaneous Removal of Cr(VI) and 4-CP
3. Results and Discussion
3.1. Characterization
3.1.1. XRD
3.1.2. BET
3.1.3. SEM
3.1.4. TEM-EDS
3.1.5. XPS
3.2. The Composition of MFNNs
3.3. Simultaneous Removal of Cr(VI) and 4-CP by Various Materials
3.4. Effect of MFNNs Dosage
3.5. Effect of Contaminant Concentrations
3.6. Effect of pH
3.7. Adsorption Isotherm and Kinetics
3.8. Effect of H2O2 Concentration
3.9. Recyclability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Fang, Z.; Kang, Y.; Tsang, E. Immobilization and phytotoxicity of chromium in contaminated soil remediated by CMC-stabilized nZVI. J. Hazard. Mater. 2014, 275, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.S.; Xu, J.; Jiang, G.M.; Tang, J.; Xu, X.H. Highly active nanoscale zero-valent iron (nZVI)-Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.N.; Zhang, X.; Chen, Z.L. Removal of chromium(VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Sabhi, S.; Kiwi, J. Degradation of 2,4-dichlorophenol by immobilized iron catalysts. Water Res. 2001, 35, 1994–2002. [Google Scholar] [CrossRef]
- Xu, J.; Lv, X.S.; Li, J.D.; Li, Y.Y.; Shen, L.; Zhou, H.Y.; Xu, X.H. Simultaneous adsorption and dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support. J. Hazard. Mater. 2012, 225–226, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Baeza, J.A.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Effect of size and oxidation state of size-controlled rhodium nanoparticles on the aqueous-phase hydrodechlorination of 4-chlorophenol. Chem. Eng. J. 2014, 240, 271–280. [Google Scholar] [CrossRef]
- Toth, A.; Torocsik, A.; Tombacz, E.; Laszlo, K. Competitive adsorption of phenol and 3-chlorophenol on purified MWCNTs. J. Colloid Interface Sci. 2012, 387, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.P.; Le, X.D.; Liu, Y.S.; Dong, C.X.; Ma, J.T. Metal organic framework derived magnetic porous carbon composite supported gold and palladium nanoparticles as highly efficient and recyclable catalysts for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. J. Mater. Chem. A 2014, 2, 18775–18785. [Google Scholar] [CrossRef]
- Yin, X.C.; Liu, W.; Ni, J.R. Removal of coexisting Cr(VI) and 4-chlorophenol through reduction and Fenton reaction in a single system. Chem. Eng. J. 2014, 248, 89–97. [Google Scholar] [CrossRef]
- Liang, D.W.; Yang, Y.H.; Xu, W.W.; Peng, S.K.; Lu, S.F.; Xiang, Y. Nonionic surfactant greatly enhances the reductive debromination of polybrominated diphenyl ethers by nanoscale zero-valent iron: Mechanism and kinetics. J. Hazard. Mater. 2014, 278, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.K.; Lai, B.; Yang, P.; Zhou, Y.X.; Wang, J.L.; Fang, S.P. Comparative study on the reactivity of Fe/Cu bimetallic particles and zero valent iron (ZVI) under different conditions of N2, air or without aeration. J. Hazard. Mater. 2015, 297, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N. Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhu, M.P.; Liu, H.L.; Ma, J.; Fang, L. Modification of Pd-Fe nanoparticles for catalytic dechlorination of 2,4-dichlorophenol. Sci. Total Environ. 2013, 449, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, M.N.; Baltazar, S.E.; García, A.; Daniela, M.L.; Pamela, S.; Maria, A.R.; Dora, A. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2015, 301, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Pan, B.C.; Zhang, W.X. Removal of selenium from water with nanoscale zero-valent iron: Mechanisms of intraparticle reduction of Se(IV). Water Res. 2015, 71, 274–281. [Google Scholar]
- Li, Y.M.; Zhang, Y.; Li, J.F.; Sheng, G.D.; Zheng, X.M. Enhanced reduction of chlorophenols by nanoscale zerovalent iron supported on organobentonite. Chemosphere 2013, 92, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Y.; Deng, S.B.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly Active and Stable Ni-Fe Bimetal Prepared by Ball Milling for Catalytic Hydrodechlorination of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Wang, J.K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H.X. Mechanism, Synthesis and Modification of Nano Zerovalent Iron in Water Treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J. Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb(II) from aqueous solution J. Mater. Chem. A 2014, 2, 15463–15472. [Google Scholar] [CrossRef]
- Magdalena, S.; Oleszczuk, P.; Yong, S.O. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2015, 287, 618–632. [Google Scholar]
- Fu, F.L.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Dror, I.; Jacov, O.M.; Cortis, A.; Berkowitz, B. Catalytic Transformation of Persistent Contaminants Using a New Composite Material Based on Nanosized Zero-Valent Iron. ACS Appl. Mater. Interfaces 2012, 4, 3416–3423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Jin, X.Y.; Chen, Z.L.; Megharaj, M.; Naidu, R. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, P.; Ma, J.; Liu, H.L.; Ning, P. Synthesis, characterization, and reactivity of cellulose modified nano zero-valent iron for dye discoloration. Appl. Surf. Sci. 2015, 345, 57–66. [Google Scholar] [CrossRef]
- Li, A.; Tai, C.; Zhao, Z.S.; Wang, Y.W.; Zhang, Q.H.; Jiang, G.B.; Hu, J.T. Debromination of Decabrominated Diphenyl Ether by Resin-Bound Iron Nanoparticles. Environ. Sci. Technol. 2007, 41, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, M.; Noubactep, C.; Gupta, V.K.; McCrindle, R.I.; Maity, A. Polyaniline/Fe0 composite nanofibers: An excellent adsorbent for the removal of arsenic from aqueous solutions. Chem. Eng. J. 2015, 271, 135–146. [Google Scholar] [CrossRef]
- Li, X.Y.; Ai, L.H.; Jiang, J. Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance. Chem. Eng. J. 2016, 288, 789–797. [Google Scholar] [CrossRef]
- Zhu, C.F.; Zeng, Z.Y.; Li, H.; Li, F.; Fan, C.H.; Zhang, H. Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J. Am. Chem. Soc. 2013, 135, 5998–6001. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Liu, B.; Matte, H.S.S.; Matte, R.; Dravid, V.P.; Rao, C.N.R. Hysteresis in Single-Layer MoS2 Field Effect Transistors. ACS Nano 2012, 6, 5635–5641. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.G.; Tolentino, L.; Yang, Z.Z.; Song, X.J.; Zhang, W.; Chen, Y.S.; Wong, C.P. High-Concentration Aqueous Dispersions of MoS2. Adv. Funct. Mater. 2013, 23, 3577–3583. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.T.; Yue, X.Y.; Yang, Q.F.; Liu, F.B.; Wang, Y.; Zhang, D.H.; Li, Z.H.; Wang, J.L. One-pot synthesis of multifunctional magnetic ferrite-MoS2-carbon dot nanohybrid adsorbent for efficient Pb(II) removal. J. Mater. Chem. A 2016, 4, 3893–3900. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.Y.; Zhang, H. Preparation and Applications of Mechanically Exfoliated Single-Layer and Multilayer MoS2 and WSe2 Nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Kim, K.H.; Jung, D.W.; Singh, K.; Oh, E.S.; Chung, J.S. Liquid phase co-exfoliated MoS2-graphene composites as anode materials for lithium ion batteries. J. Power Sources 2013, 244, 280–286. [Google Scholar] [CrossRef]
- Zhang, W.J.; Huang, J.K.; Chen, C.H.; Chang, Y.H.; Cheng, Y.J.; Li, L.J. High-Gain Phototransistors Based on a CVD MoS2 Monolayer. Adv. Funct. Mater. 2013, 25, 3456–3461. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Zhang, D.Y.; Chen, J.S.; Lou, X.W. Facile synthesis of hierarchical MoS2 microspheres composed of few-layered nanosheets and their lithium storage properties. Nanoscale 2012, 4, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.H.; He, B.Z.; Zhou, L. A general one-step approach for in situ decoration of MoS2 nanosheets with inorganic nanoparticles. J. Mater. Chem. A 2015, 3, 1042–1048. [Google Scholar] [CrossRef]
- Wang, L.C.; Ni, S.Q.; Guo, C.L.; Qian, Y.T. One pot synthesis of ultrathin boron nitride nanosheet-supported nanoscale zerovalent iron for rapid debromination of polybrominated diphenyl ethers. J. Mater. Chem. A 2013, 1, 6379–6387. [Google Scholar] [CrossRef]
- McDonnell, S.; Brennan, B.; Azcatl, A.; Lu, N.; Dong, H.; Buie, C.; Kim, J.; Hinkle, C.L.; Kim, M.J.; Wallace, R.M. HfO2 on MoS2 by Atomic Layer Deposition: Adsorption Mechanisms and Thickness Scalability. ACS Nano 2013, 7, 10354–10361. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.X. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Wang, A.Q. One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support. J. Mater. Chem. A 2015, 3, 281–289. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.Z.; Zhang, F.W.; Zhuang, Z.Y.; Yu, Y. Recyclable Nanoscale Zero Valent Iron Doped g-C3N4/MoS2 for Efficient Photocatalysis of RhB and Cr(VI) Driven by Visible Light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Li, X.Y.; Gai, F.Y.; Guan, B.; Zhang, Y.; Liu, Y.L.; Huo, Q.S. Fe@C core-shell and Fe@C yolk-shell particles for effective removal of 4-chlorophenol. J. Mater. Chem. A 2015, 3, 3988–3994. [Google Scholar] [CrossRef]
- Wang, Y.X.; Sun, H.Q.; Duan, X.G.; Ang, H.M.; Tadé, M.O.; Wang, S.B. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl. Catal. B Environ. 2015, 172–173, 73–81. [Google Scholar] [CrossRef]
- Zhu, H.J.; Jia, Y.F.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Wang, Y.H.; Chen, L.T.; Zhang, Y.; Lin, Z. Mg(OH)2 Supported Nanoscale Zero Valent Iron Enhancing the Removal of Pb(II) from Aqueous Solution. ACS Appl. Mater. Interfaces 2015, 7, 7961–7969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Tang, H.; Xue, M.Q.; Li, C.S. Facile synthesis and characterization of ultrathin MoS2 nanosheets. Mater. Lett. 2014, 130, 83–86. [Google Scholar] [CrossRef]
- Ren, Y.M.; Yan, N.; Feng, J.; Ma, J.; Wen, Q.; Li, N.; Dong, Q. Adsorption mechanism of copper and lead ions onto graphene nanosheet/δ-MnO2. Mater. Chem. Phys. 2012, 136, 538–544. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.H.; Tang, X.S.; Lu, L.; Xue, J.M. Ultrasmall Fe3O4 Nanoparticle/MoS2 Nanosheet Composites with Superior Performances for Lithium Ion Batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, S.X.; Liang, C.; Shen, S.D.; Cheng, L.; Wang, C.; Song, X.J.; Goel, S.; Barnhart, T.E.; Cai, W.B.; et al. Iron Oxide Decorated MoS2 Nanosheets with Double PEGylation for Chelator-Free Radiolabeling and Multimodal Imaging Guided Photothermal Therapy. ACS Nano 2015, 9, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; You, S.S.; Jia, X.H.; Yang, J. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment. Ceram. Int. 2015, 41, 13896–13902. [Google Scholar] [CrossRef]
- Harris, T.V.; Szilagyi, R.K. Iron-sulfur bond covalency from electronic structure calculations for classical iron-sulfur clusters. J. Comput. Chem. 2014, 35, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jeong, W.; Choe, S. Dechlorination of atrazine using zero-valent iron (Fe0) under neutral pH conditions. J. Hazard. Mater. 2008, 155, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Segura, Y.; Martínez, F.; Melero, J.; Fierro, J. Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: Treatment performance and characterization of final composites. Chem. Eng. J. 2015, 269, 298–305. [Google Scholar] [CrossRef]
- Titus, M.; Molina, V.; Banos, M.; Gimenez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lee, D.S.; Kang, N.; Yoon, J. Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Res. 1999, 33, 2110–2118. [Google Scholar] [CrossRef]
- Dombek, T.; Dolan, E.; Schultz, J.; Klarup, D. Rapid reductive dechlorination of atrazine by zero-valent iron under acidic conditions. Environ. Pollut. 2001, 111, 21–27. [Google Scholar] [CrossRef]
- Fu, H.X.; Lu, G.X.; Li, S.B. Adsorption and photo-induced reduction of Cr(VI) ion in Cr(VI)-4CP (4-chlorophenol) aqueous system in the presence of TiO2, as photocatalyst. J. Photochem. Photobiol. A Chem. 1998, 114, 81–88. [Google Scholar] [CrossRef]
- Kumar, A.; Guo, C.S.; Sharma, G.; Pathania, D.; Naushad, M.; Kalia, S.; Dhiman, P. Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(VI) and dechlorination & mineralization of 4-chlorophenol from simulated waste water. RSC Adv. 2016, 6, 13251–13263. [Google Scholar]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Yang, L.G.; Li, Q.; Zhao, J.M.; Li, X.P.; Wang, X.Q.; Liu, H.Z. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Diao, Z.H.; Xu, X.R.; Jiang, D.; Kong, L.J.; Sun, Y.X.; Hu, Y.X.; Hao, Q.W.; Chen, H. Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem. Eng. J. 2016, 302, 213–222. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Zhu, Z.J.; Yan, M.; Zhou, Y.Y.; Wang, J.J.; Liu, Y.N.; Wang, J.J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]















| nZVI Nanocomposites | Specific Surface Area (m2/g) | Reference |
|---|---|---|
| MoS2/Fe3O4/nZVI | 76.9 | - |
| g-C3N4/MoS2/nZVI | 37.5 | [43] |
| nZVI/C yolk–shell particles | 65.0 | [44] |
| Fe3C/C/nZVI | 42.3 | [45] |
| activated carbon/nZVI | 69.4 | [46] |
| Mg(OH)2/nZVI | 40.2 | [47] |
| Langmuir | Freundlich | ||
|---|---|---|---|
| qm (mg/g) | 346.34 | K (mg1−(1/n) L1/n/g) | 0.103 |
| b (L/mg) | 0.013 | n | 1.64 |
| R2 | 0.986 | R2 | 0.919 |
| Pseudo First-Order | Pseudo Second-Order | ||
|---|---|---|---|
| qe, cal (mg/g) | 327.17 | qe, cal (mg/g) | 319.88 |
| k1 (min−1) | 0.123 | k2 ((g/(mg·min)) | 0.0131 |
| R2 | 0.990 | R2 | 0.902 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Wang, J.; Hao, H.; Wang, T. Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol. Nanomaterials 2017, 7, 303. https://doi.org/10.3390/nano7100303
Lu H, Wang J, Hao H, Wang T. Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol. Nanomaterials. 2017; 7(10):303. https://doi.org/10.3390/nano7100303
Chicago/Turabian StyleLu, Haijiao, Jingkang Wang, Hongxun Hao, and Ting Wang. 2017. "Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol" Nanomaterials 7, no. 10: 303. https://doi.org/10.3390/nano7100303
APA StyleLu, H., Wang, J., Hao, H., & Wang, T. (2017). Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol. Nanomaterials, 7(10), 303. https://doi.org/10.3390/nano7100303