Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
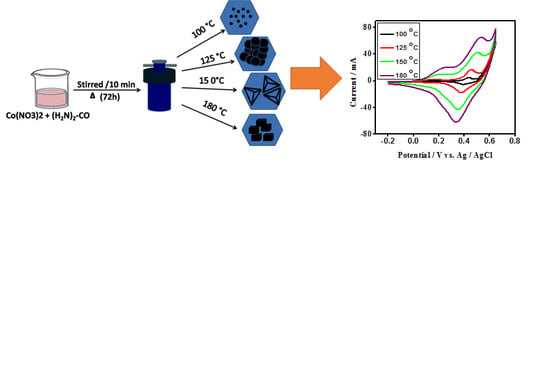
2.2. Synthesis Procedure
2.3. Characterizations
3. Results and Discussion
3.1. Physical Characterization of Co3O4 Crystallites
3.1.1. XRD Analysis
3.1.2. Infra-Red and Raman Analysis
3.1.3. Field Emission Scanning Electron Microscopy Analysis (FESEM)
3.1.4. TEM Analysis
3.2. Electrochemical Characterization
Cyclic Voltammetry (CV) of Cobalt Oxide as Working Electrode for Supercapacitor Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Hellical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Gao, L.; Zheng, H.; Zhang, Z.; Qian, Y. One-Dimensional Arrays of Co3O4 Nanoparticles: Synthesis, Characterization, and Optical and Electrochemical Properties. J. Phys. Chem. B 2004, 108, 16401–16404. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor nanowires for energy conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hasin, P.; Wu, Y. NixCo3−xO4 nanowire arrays for electrocatalytic oxygen evolution. Adv. Mater. 2010, 22, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon Nanowires: A Review on Aspects of their Growth and their Electrical Properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Yang, P.; Yan, R.; Fardy, M. Semiconductor nanowire: What’s next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasu, K.; Fuku, X.; Mola, G.T.; Manikandan, E.; Kennedy, J.; Maaza, M. Photoluminescence of well-aligned ZnO doped CeO2 nanoplatelets by a solvothermal route. Mater. Lett. 2016, 183, 351–354. [Google Scholar] [CrossRef]
- Fang, F.; Kennedy, J.; Manikandan, E.; Futter, J.; Markwitz, A. Morphology and characterization of TiO2 nanoparticles synthesized by arc discharge. Chem. Phys. Lett. 2012, 521, 86–90. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Geetha, N.; Kanimozhi, K.; Magdalene, M.; Sivaranjani, S.; Ayeshamariam, A.; Kennedy, J.; Maaza, M. In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application ny chemical method. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 Nanomaterials in Lithium-Ion Batteries and Gas Sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Maruyama, T. Electrochromic Properties of Cobalt Oxide Thin Films Prepared by Chemical Vapor Deposition. J. Electrochem. Soc. 1996, 143, 1383–1386. [Google Scholar] [CrossRef]
- Weichel, S.; Møller, P.J. Annealing-induced microfaceting of the CoO(100) surface investigated by LEED and STM. Surf. Sci. 1998, 399, 219–224. [Google Scholar] [CrossRef]
- Ramachandran, K.; Oriakhi, C.O.; Lerner, M.M.; Koch, V.R. Intercalation chemistry of cobalt and nickel dioxides: A facile route to new compounds containing organocations. Mater. Res. Bull. 1996, 31, 767–772. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Matijević, E. Colloidal cobalt hydrous oxides. Preparation and properties of monodispersed Co3O4. J. Inorg. Nucl. Chem. 1979, 41, 165–172. [Google Scholar] [CrossRef]
- Lakshmi, B.B.; Patrissi, C.J.; Martin, C.R. Sol-Gel Template Synthesis of Semiconductor Oxide Micro- and Nanostructures. Chem. Mater. 1997, 9, 2544–2550. [Google Scholar] [CrossRef]
- Yin, J.S.; Wang, Z.L. Synthesis of cobalt oxide nanocrystal self-assembled materials. J. Mater. Res. 1999, 14, 503–508. [Google Scholar] [CrossRef]
- Tarasevich, M.R.; Efremov, B.N.; Trasatti, S. Electrodes of Conductive Metallic Oxides, Part A; Elsevier: Amsterdam, The Netherlands, 1980; Chapter 5; pp. 461–462. [Google Scholar]
- Hamdani, M.; Koenig, J.F.; Chartier, P. Films minces de Co3O4 et NiCo2O4 obtenus par nebulisation reactive (spray) pour 1’electrocatalyse. II. Etude par voltamperometrie cyclique. J. Appl. Electrochem. 1988, 18, 568–576. [Google Scholar] [CrossRef]
- Singh, R.N.; Koenig, J.-F.; Poillerat, G.; Chartier, P. Electrochemical Studies on Protective Thin Co3O4 and NiCo2O4 Films Prepared on Titanium by Spray Pyrolysis for Oxygen Evolution. J. Electrochem. Soc. 1990, 137, 1408–1413. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Serizawa, M.; Sakata, H.; Hirayama, T. Electrical conductivity of Co3O4 films prepared by chemical vapour deposition. Mater. Chem. Phys. 1998, 53, 225–230. [Google Scholar] [CrossRef]
- El Baydi, M.; Poillerat, G.; Rehspringer, J.-L.; Gautier, J.L.; Koenig, J.-F.; Chartier, P. A Sol-Gel Route for the Preparation of Co3O4 Catalyst for Oxygen Electrocatalysis in Alkaline Medium. J. Solid State Chem. 1994, 109, 281–288. [Google Scholar] [CrossRef]
- Wang, R.M.; Liu, C.M.; Zhang, H.Z.; Chen, C.P.; Guo, L.; Xu, H.B.; Yang, S.H. Porous nanotubes of Co3O4: Synthesis, characterization, and magnetic properties. Appl. Phys. Lett. 2004, 85, 2080–2082. [Google Scholar] [CrossRef]
- Liu, Y.K.; Wang, G.H.; Xu, C.K.; Wang, W.Z. Fabrication of Co3O4 nanorods by calcination of precursor powders prepared in a novel inverse microemulsion. Chem. Commun. 2002, 14, 1486–1487. [Google Scholar] [CrossRef]
- Shao, W.; Yan, M. Solvothermal synthesis of cobalt oxides Nanocrystals. AIP Conf. Proc. 2017, 1846, 030004. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, H.C. Mechanistic Investigation on Salt-Mediated Formation of Free-Standing Co3O4 Nanocubes at 95 °C. J. Phys. Chem. B 2003, 107, 926–930. [Google Scholar] [CrossRef]
- Furlanetto, G.; Formaro, L. Precipitation of Spherical Co3O4 Particles. J. Colloid Interface Sci. 1995, 170, 169–175. [Google Scholar] [CrossRef]
- Feng, C.; Wang, H.; Zhang, J.; Hu, W.; Zou, Z.; Deng, Y. One-pot facile synthesis of cobalt oxide nanocubes and their magnetic properties. J. Nanopart. Res. 2014, 16, 2413. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, H.C. Size-Controlled Growth of Co3O4 Nanocubes. Chem. Mater. 2003, 15, 2829–2835. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, D.; Yin, C.; Gao, Y.; Yin, J.; Cheng, L. Nickel Foam Supported-Co3O4 Nanowire Arrays for H2O2 Electroreduction. Chem. Mater. 2009, 21, 5112–5118. [Google Scholar] [CrossRef]
- Xu, J.; Cai, J.; Wang, J.M.; Zhang, L.Y.; Fan, Y.Q.; Zhang, N.; Zhou, H.; Chen, D.; Zhong, Y.; Fan, H.Q.; et al. Facile synthesis of hierarchically porous Co3O4 nanowire arrays with enhanced electrochemical catalysis. Electrochem. Commun. 2012, 25, 119–123. [Google Scholar] [CrossRef]
- Gaddsden, J.A. Infrared Spectra of Minerals and Related Inorganic Compounds; Butterworths: London, UK, 1975; pp. 1–277. [Google Scholar]
- Meng, Y.; Chen, D.; Jiao, X. Fabrication and characterization of mesoporous Co3O4 core/mesoporous silica shell nanocomposites. J. Phys. Chem. B 2006, 110, 15212–15217. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Zeng, H.C. Interconversion of Brucite-like and Hydrotalcite-like Phases in Cobalt Hydroxide Compounds. Chem. Mater. 1999, 11, 67–74. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Song, M.; Shin, H.-S. The role of pH variation on the growth of zinc oxide nanostructures. Appl. Surf. Sci. 2009, 255, 4891–4896. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Zhang, Y.; Liu, X.; Yin, Y.; Dong, L.; Chen, S. Mesoporous β-Co(OH)2 nanowafers and naohexagonals obtained synchronously in one solution and their electrochemical hydrogen storage properties. Prog. Nat. Sci. Mater. Int. 2016, 26, 555–561. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Mitchell, D.R.G.; Issa, T.B.; Prince, K. A study of lithium insertion into MnO2 containing TiS2 additive a battery material in aqueous LiOH solution. Electrochim. Acta 2007, 52, 7007–7013. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar]
- Barmi, M.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Gong, X.; Yu, Y.; Gao, X.; Qiao, Y.; Xu, M.; Wu, H. Formation of Anhydro-sugars in the Primary Volatiles and Solid Residues from Cellulose Fast Pyrolysis in a Wire-Mesh Reactor. Energy Fuels 2014, 28, 5204–5211. [Google Scholar] [CrossRef]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials 2017, 7, 356. https://doi.org/10.3390/nano7110356
Samal R, Dash B, Sarangi CK, Sanjay K, Subbaiah T, Senanayake G, Minakshi M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials. 2017; 7(11):356. https://doi.org/10.3390/nano7110356
Chicago/Turabian StyleSamal, Rashmirekha, Barsha Dash, Chinmaya Kumar Sarangi, Kali Sanjay, Tondepu Subbaiah, Gamini Senanayake, and Manickam Minakshi. 2017. "Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications" Nanomaterials 7, no. 11: 356. https://doi.org/10.3390/nano7110356
APA StyleSamal, R., Dash, B., Sarangi, C. K., Sanjay, K., Subbaiah, T., Senanayake, G., & Minakshi, M. (2017). Influence of Synthesis Temperature on the Growth and Surface Morphology of Co3O4 Nanocubes for Supercapacitor Applications. Nanomaterials, 7(11), 356. https://doi.org/10.3390/nano7110356