Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications
Abstract
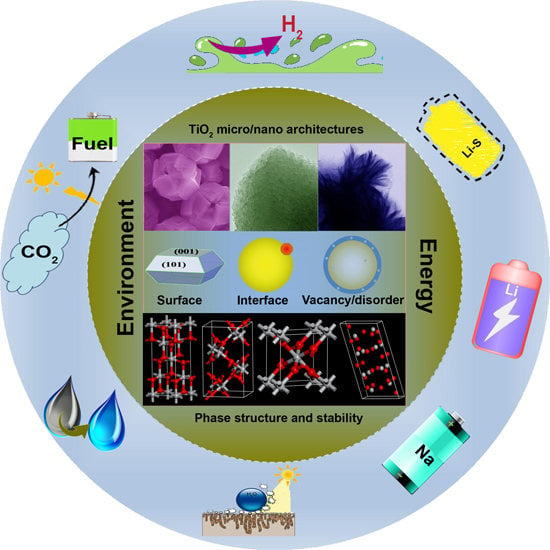
:1. Introduction
2. Advantages of Surface/Interface Engineered TiO2 Micro and Nano Structures
3. Strategies in Surface/Interface Engineering of TiO2 Micro and Nano Structures
3.1. One-Step Approach
3.2. Post Treatment Routes
3.3. Theoretical Guidance
4. Applications of Surface/Interface Engineered TiO2 Micro and Nano Structures
4.1. Photocatalysis
4.1.1. Photocatalytic Degradation of Organic Contaminants
4.1.2. Photocatalytic Hydrogen Evolution
4.1.3. Photocatalytic CO2 Reduction
4.1.4. Other Environmental Applications
4.2. Lithium/Sodium Ion Batteries
4.3. Li–S Batteries
5. Phase Stability of TiO2 Nanostructures
6. Conclusions and Perspective
- (1)
- Developing novel synthesis and treatment methods. Despite great success has been obtained in the controllable synthesis of TiO2 nanostructures with tailored micro and nano structures, there is still room for improvement in terms of quality of the products. Moreover, the new methods also provide opportunities to further understand the nucleation and growth.
- (2)
- Control of the fine structures. High-index facets and defect sites are chemically active. However, the synthesis of TiO2 nanocrystals with specific high-index facets is still a challenge. It is highly desirable to synthesize facet-controllable TiO2 materials and further study the facet effect on energy storage, conversion, and other applications. In addition, selectively generating defect structures and controlling their concentrations in different TiO2 phases are significant to revel the role of defects in various physical and chemical processes.
- (3)
- In situ/operando study the dynamic evolution of the surface/interface. In situ/operando spectroscopic or microscopic studies afford the chance to probe the evolution of TiO2 surface/interface structures in working conditions, which is crucial to study the complex phase transformation and device stability.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of novel visible light activse photocatalyst materials: Surface modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.-S.; Hagfeldt, A.; Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Ferrighi, L.; Datteo, M.; Fazio, G.; Di Valentin, C. Catalysis under cover: Enhanced reactivity at the interface between (doped) graphene and anatase TiO2. J. Am. Chem. Soc. 2016, 138, 7365–7376. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (anatase and rutile): Surface chemistry, liquid–solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Liu, Z.; Bruce, P.G.; Grey, C.P. The morphology of TiO2(B) nanoparticles. J. Am. Chem. Soc. 2015, 137, 13612–13623. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, R.; Prikryl, J.; Gärtnerova, V.; Nechvilova, K.; Benes, L.; Strizik, L.; Jäger, A.; Bosund, M.; Sopha, H.; Macak, J.M. Atomic layer deposition Al2O3 coatings significantly improve thermal, chemical, and mechanical stability of anodic TiO2 nanotube layers. Langmuir 2017, 33, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Enhancement of thermal stability of TiO2 nanowires embedded in anodic aluminum oxide template. J. Mater. Sci. 2012, 47, 739–745. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-ordered large-pore mesoporous anatase TiO2 with remarkably high thermal stability and improved crystallinity: Preparation, characterization, and photocatalytic performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Biswas, D.; Biswas, J.; Ghosh, S.; Wood, B.; Lodha, S. Enhanced thermal stability of Ti/TiO2/n-Ge contacts through plasma nitridation of TiO2 interfacial layer. Appl. Phys. Lett. 2017, 110, 052104. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, C.; Wang, Y.; Wang, S.; Dong, B.; Wan, L. Ag nanoparticle-decorated 3D flower-like TiO2 hierarchical microstructures composed of ultrathin nanosheets and enhanced photoelectrical conversion properties in dye-sensitized solar cells. J. Power Sources 2015, 292, 49–57. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Xu, Y.-F.; Rao, H.-S.; Su, C.-Y.; Kuang, D.-B. A double layered TiO2 photoanode consisting of hierarchical flowers and nanoparticles for high-efficiency dye-sensitized solar cells. Nanoscale 2013, 5, 4362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, W.; Chi, L.; Zhang, X.; Hu, W.; Jiang, B.; Pan, K.; Tian, G.; Jiang, Z. Black N/H-TiO2 nanoplates with a flower-Like hierarchical architecture for photocatalytic hydrogen evolution. ChemSusChem 2016, 9, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Lee, S.; Shin, H.; Suk Jung, H. A quasi-inverse opal layer based on highly crystalline TiO2 nanoparticles: A new light-scattering layer in dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 546–550. [Google Scholar] [CrossRef]
- King, J.S.; Graugnard, E.; Summers, C.J. TiO2 inverse opals fabricated using low-temperature atomic layer deposition. Adv. Mater. 2005, 17, 1010–1013. [Google Scholar] [CrossRef]
- Kwak, E.S.; Lee, W.; Park, N.-G.; Kim, J.; Lee, H. Compact inverse-opal electrode using non-aggregated TiO2 nanoparticles for dye-sensitized solar cells. Adv. Funct. Mater. 2009, 19, 1093–1099. [Google Scholar] [CrossRef]
- Seo, Y.G.; Woo, K.; Kim, J.; Lee, H.; Lee, W. Rapid fabrication of an inverse opal TiO2 photoelectrode for DSSC using a binary mixture of TiO2 nanoparticles and polymer microspheres. Adv. Funct. Mater. 2011, 21, 3094–3103. [Google Scholar] [CrossRef]
- Cheng, C.; Karuturi, S.K.; Liu, L.; Liu, J.; Li, H.; Su, L.T.; Tok, A.I.Y.; Fan, H.J. Quantum-dot-sensitized TiO2 inverse opals for photoelectrochemical hydrogen generation. Small 2012, 8, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-Y.; Moon, J.H. Hierarchical twin-scale inverse opal TiO2 electrodes for dye-sensitized solar cells. Langmuir 2012, 28, 9372–9377. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Du, S.; Cai, Y.; Liu, F.; Sun, P.; Zheng, J.; Lu, G. Tripartite layered photoanode from hierarchical anatase TiO2 urchin-like spheres and P25: A candidate for enhanced efficiency dye sensitized solar cells. J. Phys. Chem. C 2013, 117, 24150–24156. [Google Scholar] [CrossRef]
- Pan, J.H.; Wang, X.Z.; Huang, Q.; Shen, C.; Koh, Z.Y.; Wang, Q.; Engel, A.; Bahnemann, D.W. Large-scale synthesis of urchin-like mesoporous TiO2 hollow spheres by targeted etching and their photoelectrochemical properties. Adv. Funct. Mater. 2014, 24, 95–104. [Google Scholar] [CrossRef]
- Chen, J.S.; Liang, Y.N.; Li, Y.; Yan, Q.; Hu, X. H2O-EG-Assisted synthesis of uniform urchinlike rutile TiO2 with superior lithium storage properties. ACS Appl. Mater. Interfaces 2013, 5, 9998–10003. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Xie, B.; Pan, N.; Wang, X.; Wang, H. Novel three-dimensional dandelion-like TiO2 structure with high photocatalytic activity. J. Solid State Chem. 2008, 181, 450–456. [Google Scholar] [CrossRef]
- Musavi Gharavi, P.S.; Mohammadi, M.R. The improvement of light scattering of dye-sensitized solar cells aided by a new dandelion-like TiO2 nanostructures. Sol. Energy Mater. Sol. Cells 2015, 137, 113–123. [Google Scholar] [CrossRef]
- Lan, C.-M.; Liu, S.-E.; Shiu, J.-W.; Hu, J.-Y.; Lin, M.-H.; Diau, E.W.-G. Formation of size-tunable dandelion-like hierarchical rutile titania nanospheres for dye-sensitized solar cells. RSC Adv. 2013, 3, 559–565. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q. (Max); Cheng, H.-M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Tan, S.; Feng, H.; Cheng, Z.; Zhao, J.; Zhao, A.; Wang, B.; Luo, Y.; Yang, J.; Hou, J.G. Role of point defects on the reactivity of reconstructed anatase titanium dioxide (001) surface. Nat. Commun. 2013, 4, 2214. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, Z.; Saito, M.; Shibata, N.; Ikuhara, Y. Atomistic mechanisms of nonstoichiometry-induced twin boundary structural transformation in titanium dioxide. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, S.; Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 2016, 15, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Y.; Sushko, M.L.; Liu, J.; Luo, L.; Yoreo, J.J.D.; Mao, S.X.; Wang, C.; Rosso, K.M. Direction-specific van der Waals attraction between rutile TiO2 nanocrystals. Science 2017, 356, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Longoni, G.; Pena Cabrera, R.L.; Polizzi, S.; D’Arienzo, M.; Mari, C.M.; Cui, Y.; Ruffo, R. Shape-controlled TiO2 nanocrystals for Na-ion battery electrodes: The role of different exposed crystal facets on the electrochemical properties. Nano Lett. 2017, 17, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Guan, B.; Wang, D.; Liu, L.-M.; Lou, X.W. (David). A sulfur host based on titanium monoxide@carbon hollow spheres for advanced lithium–sulfur batteries. Nat. Commun. 2016, 7, 13065. [Google Scholar] [CrossRef] [PubMed]
- Setvín, M.; Aschauer, U.; Scheiber, P.; Li, Y.-F.; Hou, W.; Schmid, M.; Selloni, A.; Diebold, U. Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase (101). Science 2013, 341, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, H.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Modulating the photocatalytic redox preferences between anatase TiO2 {001} and {101} surfaces. Chem. Commun. 2017, 53, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Crossland, E.J.W.; Noel, N.; Sivaram, V.; Leijtens, T.; Alexander-Webber, J.A.; Snaith, H.J. Mesoporous TiO2 single crystals delivering enhanced mobility and optoelectronic device performance. Nature 2013, 495, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Pabón, B.M.; Beltrán, J.I.; Sánchez-Santolino, G.; Palacio, I.; López-Sánchez, J.; Rubio-Zuazo, J.; Rojo, J.M.; Ferrer, P.; Mascaraque, A.; Muñoz, M.C.; et al. Formation of titanium monoxide (001) single-crystalline thin film induced by ion bombardment of titanium dioxide (110). Nat. Commun. 2015, 6, 6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Schmuttenmaer, C.A. Exciton-like trap states limit electron mobility in TiO2 nanotubes. Nat. Nanotechnol. 2010, 5, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.L.; Banfield, J.F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fang, F.; Huang, G.; Sun, H.; Zhu, J.; Yu, R. Engineering the surface of rutile TiO2 nanoparticles with quantum pits towards excellent lithium storage. RSC Adv. 2016, 6, 66197–66203. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Bourret, G.R.; Berger, T.; McKenna, K.P. Modification of charge trapping at particle/particle interfaces by electrochemical hydrogen doping of nanocrystalline TiO2. J. Am. Chem. Soc. 2016, 138, 15956–15964. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Abate, A.; Correa Baena, J.P.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef] [PubMed]
- Ide, Y.; Inami, N.; Hattori, H.; Saito, K.; Sohmiya, M.; Tsunoji, N.; Komaguchi, K.; Sano, T.; Bando, Y.; Golberg, D.; et al. Remarkable charge separation and photocatalytic efficiency enhancement through interconnection of TiO2 nanoparticles by hydrothermal treatment. Angew. Chem. Int. Ed. 2016, 55, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Olds, D.; Peng, R.; Yu, L.; Foo, G.S.; Qian, S.; Keum, J.; Guiton, B.S.; Wu, Z.; Page, K. Quantitative analysis of the morphology of {101} and {001} faceted anatase TiO2 nanocrystals and its implication on photocatalytic activity. Chem. Mater. 2017, 29, 5591–5604. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, P.; Tian, Z.; Liang, C. Recent advances in surfactant-free, surface-charged, and defect-rich catalysts developed by laser ablation and processing in liquids. ChemNanoMat 2017, 3, 512–533. [Google Scholar] [CrossRef]
- Lau, M.; Straube, T.; Aggarwal, A.V.; Hagemann, U.; de Oliveira Viestel, B.; Hartmann, N.; Textor, T.; Lutz, H.; Gutmann, J.S.; Barcikowski, S. Gradual modification of ITO particle’s crystal structure and optical properties by pulsed UV laser irradiation in a free liquid jet. Dalton Trans. 2017, 46, 6039–6048. [Google Scholar] [CrossRef] [PubMed]
- Filice, S.; Compagnini, G.; Fiorenza, R.; Scirè, S.; D’Urso, L.; Fragalà, M.E.; Russo, P.; Fazio, E.; Scalese, S. Laser processing of TiO2 colloids for an enhanced photocatalytic water splitting activity. J. Colloid Interface Sci. 2017, 489, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Liang, R.; He, R.X.; Zhou, Y.N. Phase transformation of TiO2 nanoparticles by femtosecond laser ablation in aqueous solutions and deposition on conductive substrates. Nanoscale 2017, 9, 6167–6177. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, P.; Huang, W.F.; Lin, M.C. Quantum chemical elucidation of the mechanism for hydrogenation of TiO2 anatase crystals. J. Chem. Phys. 2013, 138, 154705. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.-W.; Shenoy, V.B.; Gao, H. Effects of H-, N-, and (H, N)-doping on the photocatalytic activity of TiO2. J. Phys. Chem. C 2011, 115, 12224–12231. [Google Scholar] [CrossRef]
- Aschauer, U.; Selloni, A. Hydrogen interaction with the anatase TiO2 (101) surface. Phys. Chem. Chem. Phys. 2012, 14, 16595–16602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of Anatase TiO2 by coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Edy, R.; Zhao, Y.; Huang, G.S.; Shi, J.J.; Zhang, J.; Solovev, A.A.; Mei, Y. TiO2 nanosheets synthesized by atomic layer deposition for photocatalysis. Prog. Nat. Sci. 2016, 26, 493–497. [Google Scholar] [CrossRef]
- Wang, X.; He, H.; Chen, Y.; Zhao, J.; Zhang, X. Anatase TiO2 hollow microspheres with exposed {001} facets: Facile synthesis and enhanced photocatalysis. Appl. Surf. Sci. 2012, 258, 5863–5868. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J. Photocatalytic activity of hierarchical flower-like TiO2 superstructures with dominant {001} facets. Chin. J. Catal. 2011, 32, 525–531. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Shen, Y.; Li, Z.; Wu, X.; Yan, X.; Zou, J.; Yang, S.; Zhou, W. Mesoporous black Ti3+/N-TiO2 spheres for efficient visible-light-driven photocatalytic performance. Chem. Eng. J. 2017, 325, 199–207. [Google Scholar] [CrossRef]
- An, X.; Zhang, L.; Wen, B.; Gu, Z.; Liu, L.-M.; Qu, J.; Liu, H. Boosting photoelectrochemical activities of heterostructured photoanodes through interfacial modulation of oxygen vacancies. Nano Energy 2017, 35, 290–298. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B Environ. 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Shuang, S.; Lv, R.; Xie, Z.; Zhang, Z. Surface plasmon enhanced photocatalysis of Au/Pt-decorated TiO2 nanopillar arrays. Sci. Rep. 2016, 6, 26670. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Hsu, Y.-J. Au@Cu7S4 yolk@shell nanocrystal-decorated TiO2 nanowires as an all-day-active photocatalyst for environmental purification. Nano Energy 2017, 31, 286–295. [Google Scholar] [CrossRef]
- Jin, J.; Wang, C.; Ren, X.-N.; Huang, S.-Z.; Wu, M.; Chen, L.-H.; Hasan, T.; Wang, B.-J.; Li, Y.; Su, B.-L. Anchoring ultrafine metallic and oxidized Pt nanoclusters on yolk-shell TiO2 for unprecedentedly high photocatalytic hydrogen production. Nano Energy 2017, 38, 118–126. [Google Scholar] [CrossRef]
- Yu, C.; Yu, Y.; Xu, T.; Wang, X.; Ahmad, M.; Sun, H. Hierarchical nanoflowers assembled with Au nanoparticles decorated ZnO nanosheets toward enhanced photocatalytic properties. Mater. Lett. 2017, 190, 185–187. [Google Scholar] [CrossRef]
- Wu, T.; Kang, X.; Kadi, M.W.; Ismail, I.; Liu, G.; Cheng, H.-M. Enhanced photocatalytic hydrogen generation of mesoporous rutile TiO2 single crystal with wholly exposed {111} facets. Chin. J. Catal. 2015, 36, 2103–2108. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Q.; Wang, H.; Zhang, R.; Wu, C.; Gong, J.R. TiO2 single crystal with four-truncated-bipyramid morphology as an efficient photocatalyst for hydrogen production. Small 2013, 9, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, Y.; Wang, K.; Jia, D. Green solid-state synthesis and photocatalytic hydrogen production activity of anatase TiO2 nanoplates with super heat-stability. RSC Adv. 2017, 7, 11827–11833. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.-Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, P.; Wang, Y.; Sha, L.; Ren, X.; Zhang, J.; Chen, Y.; Wu, T.; Yang, P.; Li, X. A direct charger transfer from interface to surface for the highly efficient spatial separation of electrons and holes: The construction of Ti–C bonded interfaces in TiO2-C composite as a touchstone for photocatalytic water splitting. Nano Energy 2017, 33, 29–36. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Irvine, J.T.S.; Cheng, H.-M. Enhanced photocatalytic H2 production in core-shell engineered rutile TiO2. Adv. Mater. 2016, 28, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, D.; Mubeen, S.; Chuong, T.T.; Moskovits, M.; Stucky, G.D. Anisotropic growth of TiO2 onto gold nanorods for plasmon-enhanced hydrogen production from water reduction. J. Am. Chem. Soc. 2016, 138, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Park, H.S.; Fontecilla-Camps, J.C.; Reisner, E. Photoelectrochemical H2 evolution with a hydrogenase immobilized on a TiO2-protected silicon electrode. Angew. Chem. Int. Ed. Engl. 2016, 55, 5971–5974. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Boni, A.; Melchionna, M.; Cargnello, M.; Nasi, L.; Bertoni, G.; Gorte, R.J.; Marcaccio, M.; Rapino, S.; Bonchio, M.; et al. Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution. Nat. Commun. 2016, 7, 13549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shao, M.; Xu, S.; Ning, F.; Zhou, L.; Wei, M. Photo-assisted synthesis of zinc-iron layered double hydroxides/TiO2 nanoarrays toward highly-efficient photoelectrochemical water splitting. Nano Energy 2017, 33, 21–28. [Google Scholar] [CrossRef]
- Bendova, M.; Gispert-Guirado, F.; Hassel, A.W.; Llobet, E.; Mozalev, A. Solar water splitting on porous-alumina-assisted TiO2-doped WOx nanorod photoanodes: Paradoxes and challenges. Nano Energy 2017, 33, 72–87. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. A novel architecture of dandelion-like Mo2C/TiO2 heterojunction photocatalysts towards high-performance photocatalytic hydrogen production from water splitting. J. Mater. Chem. A 2017, 5, 10591–10598. [Google Scholar] [CrossRef]
- He, H.; Lin, J.; Fu, W.; Wang, X.; Wang, H.; Zeng, Q.; Gu, Q.; Li, Y.; Yan, C.; Tay, B.K.; et al. MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600464. [Google Scholar] [CrossRef]
- Abdellah, M.; El-Zohry, A.M.; Antila, L.J.; Windle, C.D.; Reisner, E.; Hammarström, L. Time-resolved IR spectroscopy reveals a mechanism with TiO2 as a reversible electron acceptor in a TiO2–Re catalyst system for CO2 photoreduction. J. Am. Chem. Soc. 2017, 139, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Matsubu, J.C.; Zhang, S.; DeRita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2016, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, I.; Dozzi, M.V.; Bernareggi, M.; Chiarello, G.L.; Selli, E. Photocatalytic CO2 reduction vs. H2 production: The effects of surface carbon-containing impurities on the performance of TiO2-based photocatalysts. Catal. Today 2017, 281, 214–220. [Google Scholar] [CrossRef]
- Truong, Q.D.; Hoa, H.T.; Le, T.S. Rutile TiO2 nanocrystals with exposed {331} facets for enhanced photocatalytic CO2 reduction activity. J. Colloid Interface Sci. 2017, 504, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Zhou, X.; Li, Y.; Gao, Z.-D.; Song, Y.-Y.; Schmuki, P. Graphitic C3N4-sensitized TiO2 nanotube layers: A visible-light activated efficient metal-free antimicrobial platform. Chem. Eur. J. 2016, 22, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Patrocinio, A.O.T.; Paula, L.F.; Paniago, R.M.; Freitag, J.; Bahnemann, D.W. Layer-by-Layer TiO2/WO3 thin films as efficient photocatalytic self-cleaning surfaces. ACS Appl. Mater. Interfaces 2014, 6, 16859–16866. [Google Scholar] [CrossRef] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Murakami, A.; Yamaguchi, T.; Hirano, S.; Kikuta, K. Synthesis of porous titania thin films using carbonatation reaction and its hydrophilic property. Thin Solid Films 2008, 516, 3888–3892. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Wang, Q.; Zheng, A.; Zhang, Z.; Fan, J.; Liu, S.-B.; Amoureux, J.-P.; Deng, F. Understanding the high photocatalytic activity of (B, Ag)-codoped TiO2 under solar-light irradiation with XPS, solid-state NMR, and DFT calculations. J. Am. Chem. Soc. 2013, 135, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Sun, H.; Ahmad, M. 3D anatase TiO2 hollow microspheres assembled with high-energy {001} facets for lithium-ion batteries. RSC Adv. 2012, 2, 7901–7905. [Google Scholar] [CrossRef]
- Gao, X.; Li, G.; Xu, Y.; Hong, Z.; Liang, C.; Lin, Z. TiO2 Microboxes with controlled internal porosity for high-performance lithium storage. Angew. Chem. Int. Ed. Engl. 2015, 54, 14331–14335. [Google Scholar] [CrossRef] [PubMed]
- McNulty, D.; Carroll, E.; O’Dwyer, C. Rutile TiO2 inverse opal anodes for li-ion batteries with long cycle life, high-rate capability, and high structural stability. Adv. Energy Mater. 2017, 7, 1602291. [Google Scholar] [CrossRef]
- Liu, G.; Yin, L.-C.; Pan, J.; Li, F.; Wen, L.; Zhen, C.; Cheng, H.-M. Greatly enhanced electronic conduction and lithium storage of faceted TiO2 crystals supported on metallic substrates by tuning crystallographic orientation of TiO2. Adv. Mater. 2015, 27, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, H.; Han, L.; Wang, J.; Gao, P.; Xu, D.; Yang, J.; Che, S. Nanosheet-constructed porous TiO2-B for advanced lithium ion batteries. Adv. Mater. 2012, 24, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Jiao, Z.; Wang, Y.; Xu, L.; Xia, S.; Zhang, H. Eco-friendly synthesis of rutile TiO2 nanostructures with controlled morphology for efficient lithium-ion batteries. Chem. Eng. J. 2016, 304, 156–164. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Xing, H.; Gu, M.; An, J.; Zhai, B.; An, Q.; Yu, C.; Li, G. Fabrication of nest-like TiO2 hollow microspheres and its application for lithium ion batteries with high-rate performance. Electrochim. Acta 2017, 243, 112–118. [Google Scholar] [CrossRef]
- Mujtaba, J.; Sun, H.; Zhao, Y.; Xiang, G.; Xu, S.; Zhu, J. High-performance lithium storage based on the synergy of atomic-thickness nanosheets of TiO2(B) and ultrafine Co3O4 nanoparticles. J. Power Sources 2017, 363, 110–116. [Google Scholar] [CrossRef]
- Cao, M.; Gao, L.; Lv, X.; Shen, Y. TiO2-B@VS2 heterogeneous nanowire arrays as superior anodes for lithium-ion batteries. J. Power Sources 2017, 350, 87–93. [Google Scholar] [CrossRef]
- Lan, T.; Zhang, W.; Wu, N.-L.; Wei, M. Nb-doped rutile TiO2 mesocrystals with enhanced lithium storage properties for lithium ion battery. Chem.-Eur. J. 2017, 23, 5059–5065. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. 2D sandwich-like carbon-coated ultrathin TiO2@defect-rich MoS2 hybrid nanosheets: Synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 2016, 26, 541–549. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Cao, T.; He, F.; Shi, C.; He, C.; Ma, L.; Li, Q.; Li, J.; Zhao, N. Controllable graphene incorporation and defect engineering in MoS2-TiO2 based composites: Towards high-performance lithium-ion batteries anode materials. Nano Energy 2017, 33, 247–256. [Google Scholar] [CrossRef]
- Lui, G.; Li, G.; Wang, X.; Jiang, G.; Lin, E.; Fowler, M.; Yu, A.; Chen, Z. Flexible, three-dimensional ordered macroporous TiO2 electrode with enhanced electrode–electrolyte interaction in high-power Li-ion batteries. Nano Energy 2016, 24, 72–77. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.-Z.; Shu, J.; Wang, H.-E.; Li, Y.; Yu, Y.; Chen, L.-H.; Wang, B.-J.; Su, B.-L. Highly porous TiO2 hollow microspheres constructed by radially oriented nanorods chains for high capacity, high rate and long cycle capability lithium battery. Nano Energy 2015, 16, 339–349. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Liu, X.; Li, W.; Li, M. Orderly integration of porous TiO2(B) nanosheets into bunchy hierarchical structure for high-rate and ultralong-lifespan lithium-ion batteries. Nano Energy 2017, 31, 1–8. [Google Scholar] [CrossRef]
- Ren, G.; Hoque, M.N.F.; Liu, J.; Warzywoda, J.; Fan, Z. Perpendicular edge oriented graphene foam supporting orthogonal TiO2(B) nanosheets as freestanding electrode for lithium ion battery. Nano Energy 2016, 21, 162–171. [Google Scholar] [CrossRef]
- Liu, Y.; Elzatahry, A.A.; Luo, W.; Lan, K.; Zhang, P.; Fan, J.; Wei, Y.; Wang, C.; Deng, Y.; Zheng, G.; et al. Surfactant-templating strategy for ultrathin mesoporous TiO2 coating on flexible graphitized carbon supports for high-performance lithium-ion battery. Nano Energy 2016, 25, 80–90. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Quan, W.; Hong, Y.; Zhang, Z.; Tang, Z.; Li, J. Ti3+-free three-phase Li4Ti5O12/TiO2 for high-rate lithium ion batteries: Capacity and conductivity enhancement by phase boundaries. Nano Energy 2017, 32, 294–301. [Google Scholar] [CrossRef]
- Chu, S.; Zhong, Y.; Cai, R.; Zhang, Z.; Wei, S.; Shao, Z. Mesoporous and nanostructured TiO2 layer with ultra-high loading on nitrogen-doped carbon foams as flexible and free-standing electrodes for lithium-ion batteries. Small 2016, 12, 6724–6734. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.A.; Kishore, M.S.; Pralong, V.; Varadaraju, U.V.; Raveau, B. Lithium Intercalation into Nanocrystalline Brookite TiO2. Electrochem. Solid-State Lett. 2007, 10, A29–A31. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.J.; Wu, M. Ultrathin anatase TiO2 nanosheets embedded with TiO2-B nanodomains for lithium-ion storage: Capacity enhancement by phase boundaries. Adv. Energy Mater. 2015, 5, 1401756. [Google Scholar] [CrossRef]
- Mao, M.; Yan, F.; Cui, C.; Ma, J.; Zhang, M.; Wang, T.; Wang, C. Pipe-wire TiO2–Sn@carbon nanofibers paper anodes for lithium and sodium ion batteries. Nano Lett. 2017, 17, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2−x Nanotubes as a superior high-rate and ultralong-lifespan anode material for Na-ion and Li-ion batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Oschmann, B.; Buchholz, D.; Dou, X.; Lieberwirth, I.; Panthöfer, M.; Tremel, W.; Zentel, R.; Passerini, S. Extraordinary performance of carbon-coated anatase TiO2 as sodium-ion anode. Adv. Energy Mater. 2016, 6, 1501489. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-supported nanotube arrays of sulfur-doped TiO2 enabling ultrastable and robust sodium storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, Y.; Wang, C.; Li, Q.; Xiang, J.; Liang, L.; Wu, M.; Zhao, H.; Lei, Y. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano Energy 2017, 31, 514–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Foster, C.W.; Banks, C.E.; Shao, L.; Hou, H.; Zou, G.; Chen, J.; Huang, Z.; Ji, X. Graphene-rich wrapped petal-like rutile TiO2 tuned by carbon dots for high-performance sodium storage. Adv. Mater. 2016, 28, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Hou, H.; Zou, G.; Ji, X. Nitrogen doped/carbon tuning yolk-like TiO2 and its remarkable impact on sodium storage performances. Adv. Energy Mater. 2017, 7, 1600173. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen vacancies evoked blue TiO2(B) nanobelts with efficiency enhancement in sodium storage behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials Part IV. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Wei Seh, Z.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.-C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, T.; Wang, S.; Bao, Y.; Chen, G.; Ding, L.-X.; Wang, H. Free-standing sulfur host based on titanium-dioxide-modified porous-carbon nanofibers for lithium-sulfur batteries. J. Power Sources 2017, 356, 172–180. [Google Scholar] [CrossRef]
- Liang, G.; Wu, J.; Qin, X.; Liu, M.; Li, Q.; He, Y.-B.; Kim, J.-K.; Li, B.; Kang, F. Ultrafine TiO2 decorated carbon nanofibers as multifunctional interlayer for high-performance lithium–sulfur battery. ACS Appl. Mater. Interfaces 2016, 8, 23105–23113. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S. A lightweight TiO2/graphene interlayer, applied as a highly effective polysulfide absorbent for fast, long-life lithium-sulfur batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Jafta, C.J.; Lauermann, I.; Ran, Q.; Kärgell, M.; Ballauff, M.; Lu, Y. Porous Ti4O7 particles with interconnected-pore structure as a high-efficiency polysulfide mediator for lithium-sulfur batteries. Adv. Funct. Mater. 2017, 27, 1701176. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, W.; Chen, G.Z.; Cairns, E.J. Polypyrrole/TiO2 nanotube arrays with coaxial heterogeneous structure as sulfur hosts for lithium sulfur batteries. J. Power Sources 2016, 327, 447–456. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Wang, Z.; Xu, Z.-L.; Chong, W.G.; Qin, X.; Wang, X.; Kim, J.-K. Three-dimensional porous graphene aerogel cathode with high sulfur loading and embedded TiO2 nanoparticles for advanced lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 28663–28670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhao, Y.; Zu, C.; Manthiram, A. Free-standing TiO2 nanowire-embedded graphene hybrid membrane for advanced Li/dissolved polysulfide batteries. Nano Energy 2015, 12, 240–249. [Google Scholar] [CrossRef]
- Ding, B.; Xu, G.; Shen, L.; Nie, P.; Hu, P.; Dou, H.; Zhang, X. Fabrication of a sandwich structured electrode for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 14280–14285. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Structural Characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613–9644. [Google Scholar] [CrossRef] [PubMed]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of aqueous nitrobenzene by nanocrystalline TiO2. Ind. Eng. Chem. Res. 2006, 45, 922–927. [Google Scholar] [CrossRef]
- Wagner, J.B.; Cavalca, F.C.; Damsgaard, C.D.; Duchstein, L.D.L.; Hansen, T.W.; Renu Sharma, P.A.C. Exploring the environmental transmission electron microscope. Micron 2012, 43, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Wang, Y.; Li, H.; Wu, H.; Zhang, Z.; Selloni, A.; Sun, C. Real-time observation of reconstruction Dynamics on TiO2(001) surface under oxygen via an environmental transmission electron microscope. Nano Lett. 2016, 16, 132–137. [Google Scholar] [CrossRef] [PubMed]











| Material/[Reference] | Capacity (Cycles) (mA·h·g−1) | Rate Capability (mA·h·g−1) | Voltage (V) |
|---|---|---|---|
| Rutile TiO2 with quantum pits [50] | 145 (80)@168 mA·g−1 | 102@1675 mA·g−1 | 1–3/Li |
| TiO2 microboxes [106] | 187 (300)@170 mA·g−1 | 63@3400 mA·g−1 | 1–3/Li |
| Rutile TiO2 inverse opals [107] | 95 (5000)@450 mA·g−1 | - | 1–3/Li |
| Faceted TiO2 crystals [108] | 141.2 (100)@170 mA·g−1 | 29.9@1700 mA·g−1 | 1–3/Li |
| Nanosheet-constructed TiO2(B) [109] | 200 (200)@3350 mA·g−1 | 216@3350 mA·g−1 | 1–3/Li |
| TiO2 hollow microspheres [105] | 157 (50)@170 mA·g−1 | 90@1700 mA·g−1 | 1–3/Li |
| rutile TiO2 nanostructures [110] | 190 (200)@102 mA·g−1 | 84.5@1700 mA·g−1 | 1–3/Li |
| nest-like TiO2 hollow microspheres [111] | 152 (100)@1020 mA·g−1 | 130@3400 mA·g−1 | 1–3/Li |
| Co3O4 NPs@TiO2(B) NSs [112] | 677.3 (80)@100 mA·g−1 | 386@1000 mA·g−1 | 0.01–3.0/Li |
| TiO2(B)@VS2 nanowire arrays [113] | 365.4 (500)@335 mA·g−1 | 171.2@3350 mA·g−1 | 0.01–3.0/Li |
| Nb-doped rutile TiO2 Mesocrystals [114] | 141.9 (600)@850 mA·g−1 | 96.3@6800 mA·g−1 | 1–3/Li |
| TiO2@defect-rich MoS2 nanosheets [115] | 805.3 (100)@100 mA·g−1 | 507.6@2000 mA·g−1 | 0.005–3.0/Li |
| MoS2-TiO2 based composites [116] | 648 (400)@1000 mA·g−1 | 511@2000 mA·g−1 | 0.005–3.0/Li |
| macroporous TiO2 [117] | 181 (1000)@1700 mA·g−1 | [email protected] A·g−1 | 1–3/Li |
| porous TiO2 hollow microspheres [118] | 216 (100)@170 mA·g−1 | 112@1700 mA·g−1 | 1–3/Li |
| porous TiO2(B) nanosheets [119] | 186 (1000)@1675 mA·g−1 | 159@6700 mA·g−1 | 1–3/Li |
| graphene supported TiO2(B) sheets [120] | 325 (10000)@500 mA·g−1 | 49@40 A·g−1 | 1–3/Li |
| mesoporous TiO2 coating on carbon [121] | 210 (1000)@3400 mA·g−1 | [email protected] A·g−1 | 1–3/Li |
| Ti3+-free three-phase Li4Ti5O12/TiO2 [122] | 136 (1000)@4000 mA·g−1 | 155.6@8 A·g−1 | 1.0–2.5/Li |
| Mesoporous TiO2 [123] | 149 (100)@1000 mA·g−1 | 104@2000 mA·g−1 | 1–3/Li |
| Nanocrystalline brookite TiO2 [124] | 170 (40)@35 mA·g−1 | - | 1–3/Li |
| Anatase TiO2 embedded with TiO2(B) [125] | 190 (1000)@1700 mA·g−1 | 110@8500 mA·g−1 | 1–3/Li |
| TiO2-Sn@carbon nanofibers [126] | 413 (400)@100 mA·g−1 | - | 0.01–2.0/Na |
| Double-walled Sb@TiO2-x nanotubes [127] | 300 (1000)@2.64 A·g−1 | [email protected] A·g−1 | 0.1–2.5/Na |
| Carbon-coated anatase TiO2 [128] | 180 (500)@1675 mA·g−1 | [email protected] A·g−1 | 0.05–2.0/Na |
| Nanotube arrays of S-doped TiO2 [129] | 136 (4400)@3350 mA·g−1 | 167@3350 mA·g−1 | 0.1–2.5/Na |
| Amorphous TiO2 inverse opal [130] | 203 (100)@100 mA·g−1 | 113@5 A·g−1 | 0.01–3.0/Na |
| Petal-like rutile TiO2 [131] | 144.4 (1100)@837.5 mA·g−1 | 59.8@4187 mA·g−1 | 0.01–3.0/Na |
| Yolk-like TiO2 [132] | 200.7 (550)@335 mA·g−1 | 90.6@8375 mA·g−1 | 0.01–3.0/Na |
| Blue TiO2(B) nanobelts [133] | 210.5 (5000)@3350 mA·g−1 | 90.6@5025 mA·g−1 | 0.01–3.0/Na |
| Material/[Reference] | Capacity (Cycles) (mA·h·g−1) | Rate Capability (mA·h·g−1) | Sulfur Loading (%) | Voltage (V) |
|---|---|---|---|---|
| TiO@carbon [40] | 750 (500)@335 mA·g−1 | 655 @3.35 A·g−1 | ~70 | 1.9–2.6 |
| Ti4O7/S [135] | 1070 (500)@3350 mA·g−1 | - | 70 | 1.8–3.0 |
| TiO2/N-doped graphene [136] | 918 (500)@1675 mA·g−1 | 833 @6.7 A·g−1 | 59 | 1.7–2.8 |
| S–TiO2 yolk–shell [137] | 1030 (1000)@837 mA·g−1 | 630 @3.35 A·g−1 | 62 | 1.7–2.6 |
| TiO2-porous carbon nanofibers [138] | 618 (500)@1675 mA·g−1 | 668 @8.375 A·g−1 | 55 | 1.7–2.6 |
| TiO2-carbon nanofibers [139] | 694 (500)@1675 mA·g−1 | 540 @3.35 mA·g−1 | 68.83 | 1.7–2.8 |
| TiO2/graphene [140] | 630 (1000)@3350 mA·g−1 | 535 @5.025 A·g−1 | 51.2 | 1.6–2.8 |
| Porous Ti4O7 particles [141] | 989 (300)@167.5 mA·g−1 | 873 @1.675 A·g−1 | 50-55 | 1.8–3.0 |
| Polypyrrole/TiO2 nanotube arrays [142] | 1150 (100)@167.5 mA·g−1 | - | 61.93 | 1.8–3.0 |
| Graphene-TiO2 NPs [143] | 663 (100)@1675 mA·g−1 | - | 75 | 1.7–2.8 |
| TiO2 nanowire/graphene [144] | 1053 (200)@335 mA·g−1 | - | 60 | 1.5–2.8 |
| graphene/TiO2/S [145] | 597 (100)@1675 mA·g−1 | - | 60 | 1.5–3.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, Y.; Mølhave, K.; Sun, H. Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications. Nanomaterials 2017, 7, 382. https://doi.org/10.3390/nano7110382
Wang X, Zhao Y, Mølhave K, Sun H. Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications. Nanomaterials. 2017; 7(11):382. https://doi.org/10.3390/nano7110382
Chicago/Turabian StyleWang, Xiaoliang, Yanyan Zhao, Kristian Mølhave, and Hongyu Sun. 2017. "Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications" Nanomaterials 7, no. 11: 382. https://doi.org/10.3390/nano7110382
APA StyleWang, X., Zhao, Y., Mølhave, K., & Sun, H. (2017). Engineering the Surface/Interface Structures of Titanium Dioxide Micro and Nano Architectures towards Environmental and Electrochemical Applications. Nanomaterials, 7(11), 382. https://doi.org/10.3390/nano7110382