Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application
Abstract
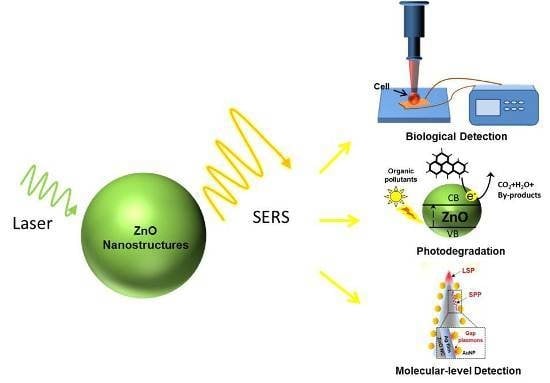
:1. Introduction
2. Improved SERS Activity of ZnO Nanostructures: Theoretical Basis and Improved Methods
2.1. Theoretical Basis
2.1.1. Theoretical Basis for Improving the Electromagnetic Enhancement of Semiconductor ZnO
2.1.2. Theoretical Basis for Improving Chemical Enhancement of Semiconductor ZnO
2.2. Improvement Methods
3. A Unique Advantage of Semiconductor ZnO as SERS Substrate
4. Necessity of Reporting a Meaningful Average EF
5. Synthesis of ZnO Nanostructures as SERS Substrates
5.1. Synthesis of 0-D ZnO Nanostructures
5.1.1. Synthesis of ZnO Nanospheres
5.1.2. Synthesis of ZnO Nanocages
5.2. Synthesis of 1-D ZnO Nanostructures
5.3. Synthesis of 2-D ZnO Nanostructures
5.3.1. Synthesis of ZnO Nanosheets
5.3.2. Synthesis of ZnO Film
5.4. Synthesis of 3-D ZnO Nanostructures
5.4.1. Synthesis of ZnO Nanorod Arrays
5.4.2. Synthesis of 3-D Sandwich Structure Assembly
6. ZnO Nanostructures for Versatile SERS Application
6.1. Pure ZnO Nanostructure Materials
6.1.1. Morphology Optimization Design of Pure ZnO Substrates
6.1.2. Structure Optimization Design of Pure ZnO Substrates
6.1.3. Size Optimization Design of Pure ZnO Substrates
6.1.4. Effects of Crystallinity on the SERS Activity of Pure ZnO Substrates
6.1.5. Prerequisite for Realizing SERS on Pure ZnO Substrates: PICT
6.2. Elemental Doped ZnO Nanomaterials
6.2.1. Effects of Doping Concentration on SERS Activity of Elemental Doped ZnO Substrates
6.3. Noble Metal/ZnO Composite Materials
6.3.1. Ag/ZnO Composite Materials
6.3.2. Au/ZnO Composite Materials
6.3.3. ZnO/Ag/Au Composite Materials
6.4. 3-D-Sandwich Structure Nanomaterials
6.4.1. Noble Metal/Molecules/ZnO 3-D-Sandwich Structural Composite Substrates
6.4.2. Graphene/Noble Metal/ZnO 3-D-Sandwich Structural Composite Substrates
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Griffith Freeman, R.; Grabar, K.C.; Allison, K.J.; Bright, R.M.; Davis, J.A.; Guthrie, A.P.; Hommer, M.B.; Jackson, M.A.; Smith, P.C.; Walter, D.G.; et al. Self-Assembled Metal Colloid Monolayers: An Approach to SERS Substrates. Science 1995, 267, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.A.; Moore, D.S. Progress in plasmonic engineering of surface-enhanced Raman-scattering substrates toward ultra-trace analysis. Anal. Bioanal. Chem. 2005, 382, 1751–1770. [Google Scholar] [CrossRef] [PubMed]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridzne adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.; Creighton, J. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Qian, X.M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tanemura, M.; Huang, Z.; Jiang, D.; Li, Z.Y.; Huang, Y.P.; Kawamura, G.; Yamaguchi, K.; Nogami, M. Aligned gold nanoneedle arrays for surface-enhanced Raman scattering. Nanotechnology 2010, 21, 325701. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; McKeating, K.S.; Faulds, K. Recent developments and future directions in SERS for bioanalysis. Phys. Chem. Chem. Phys. 2013, 15, 5312–5328. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, A.; Wang, D.; Guo, H.; Sun, H.; He, Q. Fabrication of cube-like Fe3O4@SiO2@Ag nanocomposites with high SERS activity and their application in pesticide detection. J. Nanopart. Res. 2016, 18, 178. [Google Scholar] [CrossRef]
- Hurst, S.J.; Fry, H.C.; Gosztola, D.J.; Rajh, T. Utilizing Chemical Raman Enhancement: A Route for Metal Oxide Support-Based Biodetection. J. Phys. Chem. C 2011, 115, 620–630. [Google Scholar] [CrossRef]
- Brus, L. Noble Metal Nanocrystals: Plasmon Electron Transfer Photochemistry and Single-Molecule Raman Spectroscopy. Acc. Chem. Res. 2008, 41, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chon, H.; Yoon, S.-Y.; Lee, E.K.; Chang, S.-I.; Lim, D.W.; Choo, J. Fabrication of SERS-fluorescence dual modal nanoprobes and application to multiplex cancer cell imaging. Nanoscale 2012, 4, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhou, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. 3D Fe3O4@Au@Ag Nanoflowers Assembled Magnetoplasmonic Chains for in situ SERS Monitoring of Plasmon-assisted Catalytic Reaction. J. Mater. Chem. A 2016, 4, 8866–8874. [Google Scholar] [CrossRef]
- Kundu, S.; Mandal, M.; Ghosh, S.K.; Pal, T. Photochemical deposition of SERS active silver nanoparticles on silica gel and their application as catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2004, 272, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hakonen, A.; Wang, F.; Andersson, P.O.; Wingfors, H.; Rindzevicius, T.; Schmidt, M.S.; Soma, V.R.; Xu, S.; Li, Y.; Boisen, A.; et al. Hand-Held Femtogram Detection of Hazardous Picric Acid with Hydrophobic Ag Nanopillar SERS Substrates and Mechanism of Elasto-Capillarity. ACS Sens. 2017, 2, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.S.R.; Singh, A.K.; Senapati, D.; Yu, H.; Ray, P.C. Gold Nanoparticle Based Label-Free SERS Probe for Ultrasensitive and Selective Detection of Trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Dai, Z.; Tian, Q.; Liu, J.; Xiao, X.; Jiang, C.; Wu, W.; Roy, V.A.L. Wetting properties and SERS applications of ZnO/Ag nanowire arrays patterned by a screen printing method. J. Mater. Chem. C 2016, 4, 6371–6379. [Google Scholar] [CrossRef]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface Enhanced Raman Spectroscopy of Individual Rhodamine 6G Molecules on Large Ag Nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Zeman, E.J.; Schatz, G.C. An accurate electromagnetic theory study of surface enhancement factors for silver, gold, copper, lithium, sodium, aluminum, gallium, indium, zinc, and cadmium. J. Phys. Chem. 1987, 91, 634–643. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Nie, S. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bjerneld, E.; Käll, M.; Börjesson, L. Spectroscopy of Single Hemoglobin Molecules by Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Yang, Y.; Huang, Z.; Long, N.V.; Fu, C. Engineering of SERS Substrates Based on Noble Metal Nanomaterials for Chemical and Biomedical Applications. Appl. Spectrosc. Rev. 2015, 50, 499–525. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.-Y.; Yamaguchi, K.; Tanemura, M.; Huang, Z.; Jiang, D.; Chen, Y.; Zhou, F.; Nogami, M. Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale 2012, 4, 2663. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, W.; She, G.; Mu, L. Surface-Enhanced Raman Scattering (SERS) on transition metal and semiconductor nanostructures. Phys. Chem. Chem. Phys. 2012, 14, 5891–5901. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Song, W.; Tanabe, I.; Wang, Y.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering for highly robust SERS sensing: The case of phosphate analysis. Chem. Commun. 2015, 51, 7641–7644. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yamamoto, Y. Surface enhanced Raman scattering (SERS) of chemisorbed species on various kinds of metals and semiconductors. Surf. Sci. 1983, 134, 71–90. [Google Scholar] [CrossRef]
- Hayashi, S.; Koh, R.; Ichiyama, Y.; Yamamoto, K. Evidence for surface-enhanced Raman scattering on nonmetallic surfaces: Copper phthalocyanine molecules on GaP small particles. Phys. Rev. Lett. 1988, 60, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.A.O. Surface enhancement of Raman and absorption spectra from cyanine dye D266 adsorbed on ZnO colloids. Mol. Phys. 1996, 88, 281–290. [Google Scholar] [CrossRef]
- Shan, Y.; Zheng, Z.; Liu, J.; Yang, Y.; Li, Z.; Huang, Z.; Jiang, D. Niobium pentoxide: A promising surface-enhanced Raman scattering active semiconductor substrate. NPJ Comp. Mater. 2017, 3, 11. [Google Scholar] [CrossRef]
- Quagliano, L.G. Observation of Molecules Adsorbed on III-V Semiconductor Quantum Dots by Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2004, 126, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor materials in analytical applications of surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 51–58. [Google Scholar] [CrossRef]
- Tan, X.; Melkersson, J.; Wu, S.; Wang, L.; Zhang, J. Noble-Metal-Free Materials for Surface-Enhanced Raman Spectroscopy Detection. Chemphyschem 2016, 17, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, H.; Li, G. Metal oxide semiconductor SERS-active substrates by defect engineering. Analyst 2017, 142, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Liao, F.; Liu, S.; Zhao, Y.; Kang, Z.; Zhang, X.; Shao, M. Bi-functional ZnO–RGO–Au substrate: Photocatalysts for degrading pollutants and SERS substrates for real-time monitoring. Chem. Commun. 2013, 49, 3049. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Yang, Y.; Cao, Y.; Huang, Z. Facile solvothermal synthesis of Ag/Fe3O4 nanocomposites and their SERS applications in on-line monitoring of pesticide contaminated water. RCS Adv. 2015, 5, 102610–102618. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Wang, Q.; Duo, H.; Zhang, X.; Li, Y.; Huang, W. Photocatalytically Renewable Micro-electrochemical Sensor for Real-Time Monitoring of Cells. Angew. Chem. Int. Ed. 2015, 54, 14402–14406. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Pereira, L.M.N.; Martins, R.F.P. Fully Transparent ZnO Thin-Film Transistor Produced at Room Temperature. Adv. Mater. 2005, 17, 590–594. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanobelts, Nanowires, and Nanodiskettes of Semiconducting Oxides—From Materials to Nanodevices. Adv. Mater. 2003, 15, 432–436. [Google Scholar] [CrossRef]
- Vernardou, D.; Kenanakis, G.; Couris, S.; Koudoumas, E.; Kymakis, E.; Katsarakis, N. pH effect on the morphology of ZnO nanostructures grown with aqueous chemical growth. Thin Solid Films 2007, 515, 8764–8767. [Google Scholar] [CrossRef]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; McKenzie, B.; McDermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Pearton, S. Recent progress in processing and properties of ZnO. Prog. Mater. Sci. 2005, 50, 293–340. [Google Scholar] [CrossRef]
- Hochbaum, A.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R.; Surya, S. Facile growth of ZnO nanowire arrays and nanoneedle arrays with flower structure on ZnO-TiO2 seed layer for DSSC applications. J. Alloys Compd. 2017, 693, 1011–1019. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y.; Yu, K.; Tang, X.; Zhang, Y.; Wang, S.; Wei, C. Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 2016, 6, 35079. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hogan, T.P.; Shanker, B. Gold-coated zinc oxide nanowire-based substrate for surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 1539–1545. [Google Scholar] [CrossRef]
- Li, Y.; Cai, W.; Duan, G.; Cao, B.; Sun, F.; Lu, F. Superhydrophobicity of 2D ZnO ordered pore arrays formed by solution-dipping template method. J. Colloid Interface Sci. 2005, 287, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Bagra, B.; Pimpliskar, P.; Agrawal, N.K. Bio-compatibility, surface & chemical characterization of glow discharge plasma modified ZnO nanocomposite polycarbonate. Soild State Phys. 2014, 189–191. [Google Scholar] [CrossRef]
- Metiu, H. Surface enhanced spectroscopy. Prog. Surf. Sci. 1984, 17, 153–320. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Nitzan, A.; Brus, L.E. Theoretical model for enhanced photochemistry on rough surfaces. J. Chem. Phys. 1981, 75, 2205–2214. [Google Scholar] [CrossRef]
- Shegai, T.; Vaskevich, A.; Rubinstein, I.; Haran, G. Raman Spectroelectrochemistry of Molecules within Individual Electromagnetic Hot Spots. J. Am. Chem. Soc. 2009, 131, 14390–14398. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Aizpurua, J.; Kall, M.; Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys. Rev. E 2000, 62, 4318–4324. [Google Scholar] [CrossRef]
- Lee, P.C.; Meise, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Sco. Rev. 1998, 27, 241. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-Enhanced Raman Scattering: Active Nanomaterials and Applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Van de Walle, C.G. Computationally predicted energies and properties of defects in GaN. NPJ Comp. Mater. 2017, 3, 12. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Grand, J.; Sow, I.; Somerville, W.R.C.; Etchegoin, P.G.; Treguer-Delapierre, M.; Charron, G.; Félidj, N.; Lévi, G.; Aubard, J.; et al. A scheme for detecting every single target molecule with surface-enhanced Raman spectroscopy. Nano Lett. 2011, 11, 5013–5019. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Drude, P. Zur Elektronentheorie der Metalle. Ann. Phys. 1990, 306, 566–613. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B. Noble-metal-free plasmonic photocatalyst: Hydrogen doped semiconductors. Sci. Rep. 2014, 4, 3986. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Moura, L.G.; Pimenta, M.A.; Zhang, J. Charge-Transfer Mechanism in Graphene-Enhanced Raman Scattering. J. Phys. Chem. C 2012, 116, 25112–25118. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Time-dependent picture of the charge-transfer contributions to surface enhanced Raman spectroscopy. J. Chem. Phys. 2007, 126, 244709. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2 Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040–6041. [Google Scholar] [CrossRef] [PubMed]
- Fonoberov, V.A.; Alim, K.A.; Balandin, A.A.; Xiu, F.; Liu, J. Photoluminescence investigation of the carrier recombination processes in ZnO quantum dots and nanocrystals. Phys. Rev. B 2006, 73, 165317. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, W.; Zhang, J.; Yang, B.; Xu, W.; Zhao, B.; Lombardi, J.R. Direct observation of surface-enhanced Raman scattering in ZnO nanocrystals. J. Raman Spectrosc. 2009, 40, 1072–1077. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Ren, X.; Tang, J.; Li, Y.; Zhang, X.; Cheng, Z. Au–ZnO hybrid nanoparticles exhibiting strong charge-transfer-induced SERS for recyclable SERS-active substrates. Nanoscale 2015, 7, 5147–5151. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.-H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzán, L.M. Growth and galvanic replacement of silver nanocubes in organic media. Nanoscale 2013, 5, 4355. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.-B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanooctahedra with Tunable Size and Microfluidic-Induced 3D Assembly for Highly Uniform SERS-Active Supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Fernández-López, C.; Polavarapu, L.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Contreras-Cáceres, R.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanorod–pNIPAM Hybrids with Reversible Plasmon Coupling: Synthesis, Modeling, and SERS Properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Gasymov, O.K.; Alekperov, O.Z.; Aydemirova, A.H.; Kamilova, N.; Aslanov, R.B.; Bayramov, A.H.; Kerimova, A. Surface enhanced Raman scattering of whole human blood on nanostructured ZnO surface. Phys. Status Solidi C 2017, 14, 1600155. [Google Scholar]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Hakonen, A.; Svedendahl, M.; Ogier, R.; Yang, Z.-J.; Lodewijks, K.; Verre, R.; Shegai, T.; Andersson, P.O.; Käll, M. Dimer-on-mirror SERS substrates with attogram sensitivity fabricated by colloidal lithography. Nanoscale 2015, 7, 9405–9410. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ruan, W.; Yang, L.; Ji, W.; Xie, Y.; Chen, L.; Song, W.; Zhao, B.; Lombardi, J.R. Surface-enhanced Raman scattering of molecules adsorbed on Co-doped ZnO nanoparticles. J. Raman Spectrosc. 2012, 43, 61–64. [Google Scholar] [CrossRef]
- Chang, L.; Xu, D.; Xue, X. Photoluminescence and Raman scattering study in ZnO:Mg nanocrystals. J. Mater. Sci. Mater. Electron. 2015, 27, 1014–1019. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, B.; Lombardi, J.R. ZnO nanoparticle size-dependent excitation of surface Raman signal from adsorbed molecules: Observation of a charge-transfer resonance. Appl. Phys. Lett. 2007, 91, 221106. [Google Scholar] [CrossRef]
- Richter, A.P.; Lombardi, J.R.; Zhao, B. Size and Wavelength Dependence of the Charge-Transfer Contributions to Surface-Enhanced Raman Spectroscopy in Ag/PATP/ZnO Junctions. J. Phys. Chem. C 2010, 114, 1610–1614. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Z.; Shang, J.; Sun, X.; Cai, W.; Guo, H. The preparation and characterization of ZnO ultrafine particles. Mater. Sci. Eng. A 2002, 332, 356–361. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Hu, H.; Zhao, B. Fabrication of surface-enhanced Raman scattering-active ZnO/Ag composite microspheres. J. Raman Spectrosc. 2007, 38, 1320–1325. [Google Scholar] [CrossRef]
- Seelig, E.W.; Tang, B.; Yamilov, A.; Cao, H.; Chang, R.P.H. Self-assembled 3D photonic crystals from ZnO colloidal spheres. Mater. Chem. Phys. 2003, 80, 257–263. [Google Scholar] [CrossRef]
- Jezequel, D.; Guenot, J.; Jouini, N.; Fievet, F. Preparation and Morphological Characterization of Fine, Spherical, Monodisperse Particles of ZnO. Mater. Sci. Forum 1994, 152–153, 339–342. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Z.; Huang, W.; Lin, J.; Ma, G.; Li, S.; Guo, L. Remarkable SERS Activity Observed from Amorphous ZnO Nanocages. Angew. Chem. Int. Ed. 2017, 56, 9851–9855. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Peng, J.-W.; Liu, C.-S. Photoluminescence and SERS investigation of plasma treated ZnO nanorods. Appl. Surf. Sci. 2013, 285, 748–754. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, P.; Navrotsky, A.; Yuan, Z.; Ren, T.; Halasa, M.; Su, B. Hierarchically Assembled Porous ZnO Nanoparticles: Synthesis, Surface Energy, and Photocatalytic Activity. Chem. Mater. 2007, 19, 5680–5686. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Guo, L. Precursor-Directed Self-Assembly of Porous ZnO Nanosheets as High-Performance Surface-Enhanced Raman Scattering Substrate. Small 2014, 10, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Zhang, T.; Dong, W.; Wei, T.; Sun, Y.; Chen, X.; Shen, G.; Dai, N. Vertically coupled ZnO nanorods on MoS2 monolayers with enhanced Raman and photoluminescence emission. Nano Res. 2014, 8, 743–750. [Google Scholar] [CrossRef]
- Wu, S.; Huang, C.; Aivazian, G.; Ross, J.S.; Cobden, D.H.; Xu, X. Vapor Solid Growth of High Optical Quality MoS2 Monolayers with Near-Unity Valley Polarization. ACS Nano 2013, 7, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Jayram, N.D.; Sonia, S.; Poongodi, S.; Kumar, P.S.; Masuda, Y.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Superhydrophobic Ag decorated ZnO nanostructured thin film as effective surface enhanced Raman scattering substrates. Appl. Surf. Sci. 2015, 355, 969–977. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, Y.; Cao, Y.; Fu, C.; Huang, Z. Synthesis of wheatear-like ZnO nanoarrays decorated with Ag nanoparticles and its improved SERS performance through hydrogenation. Nanotechnology 2016, 27, 145502. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Lee, T.K.; Park, J.; Ha, M.; Kwak, S.K.; Ko, H. Particle-on-Film Gap Plasmons on Antireflective ZnO Nanocone Arrays for Molecular-Level Surface-Enhanced Raman Scattering Sensors. ACS Appl. Mater. Interfaces 2015, 7, 26421–26429. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; She, G.; Xu, H.; Mu, L.; Shi, W. The surface-enhanced Raman scattering from ZnO nanorod arrays and its application for chemosensors. Sens. Actutator B Chem. 2014, 193, 745–751. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.L.; Shin, K.S. Raman spectral characteristics of 4-aminobenzenethiol adsorbed on ZnO nanorod arrays. Phys. Chem. Chem. Phys. 2013, 15, 9288–9294. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-Temperature Wafer-Scale Production of ZnO Nanowire Arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-Assembly of ZnO: From Nanodots to Nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Zhang, W.-D. Growth of ZnO nanowires on modified well-aligned carbon nanotube arrays. Nanotechnology 2006, 17, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yan, B.; Wong, S.M.; Li, X.; Zhou, W.; Yu, T.; Shen, Z.; Yu, H.; Fan, H.J. Fabrication and SERS Performance of Silver-Nanoparticle-Decorated Si/ZnO Nanotrees in Ordered Arrays. ACS Appl. Mater. Interfaces 2010, 2, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Q.; Duo, H.-H.; Zhang, Y.-G.; Zhang, X.-W.; Fang, W.; Liu, Y.-L.; Shen, A.-G.; Hu, J.-M.; Huang, W.-H. Photochemical Synthesis of Shape-Controlled Nanostructured Gold on Zinc Oxide Nanorods as Photocatalytically Renewable Sensors. Anal. Chem. 2016, 88, 3789–3795. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, C.; Lu, J.; Li, Z.; Tian, Z. 3D Ag/ZnO hybrids for sensitive surface-enhanced Raman scattering detection. Appl. Surf. Sci. 2016, 365, 291–295. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, S.; Mao, Z.; Li, P.; Zhao, C.; Cohick, Z.; Huang, P.-H.; Huang, T.J. In Situ Fabrication of 3D Ag@ZnO Nanostructures for Microfluidic Surface-Enhanced Raman Scattering Systems. ACS Nano 2014, 8, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Song, W.; Xue, X.; Ji, W.; Li, Z.; Chen, L.; Mao, H.; Lv, H.; Wang, X.; Lombardi, J.R.; et al. Interfacial Charge-Transfer Effects in Semiconductor–Molecule–Metal Structures: Influence of Contact Variation. J. Phy. Chem. C 2012, 116, 14701–14710. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, C.; Yang, J.; Zhao, B.; Lombardi, J.R. Nanoparticle Metal-Semiconductor Charge Transfer in ZnO/PATP/Ag Assemblies by Surface-Enhanced Raman Spectroscopy. J. Phy. Chem. C 2008, 112, 6093–6098. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Shim, E.-L.; Choi, Y.-J.; Park, J.-H.; Yoon, S. Giant Enhancement of Raman Response Due to OneDimensional ZnO Nanostructures. Nanoscale 2014, 6, 14622–14626. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; She, G.; Wang, X.; Mu, L.; Shi, W. Enhancing the SERS performance of semiconductor nanostructures through a facile surface engineering strategy. Appl. Surf. Sci. 2014, 320, 591–595. [Google Scholar] [CrossRef]
- Song, W.; Ji, W.; Vantasin, S.; Tanabe, I.; Zhao, B.; Ozaki, Y. Fabrication of a highly sensitive surface-enhanced Raman scattering substrate for monitoring the catalytic degradation of organic pollutants. J. Mater. Chem. A 2015, 3, 13556–13562. [Google Scholar] [CrossRef]
- He, X.; Yue, C.; Zang, Y.; Yin, J.; Sun, S.; Li, J.; Kang, J. Multi-hot spot configuration on urchin-like Ag nanoparticle/ZnO hollow nanosphere arrays for highly sensitive SERS. J. Mater. Chem. A 2013, 1, 15010. [Google Scholar] [CrossRef]
- Sinha, G.; Depero, L.E.; Alessandri, I. Recyclable SERS Substrates Based on Au-Coated ZnO Nanorods. ACS Appl. Mater. Interfaces 2011, 3, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Zhu, R.; Guo, X. Nanostructured gold microelectrodes for SERS and EIS measurements by incorporating ZnO nanorod growth with electroplating. Sci. Rep. 2015, 5, 16454. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ruan, W.; Jiang, X.; Zhao, B.; Xu, W.; Lombardi, J.R. Contribution of ZnO to Charge-Transfer Induced Surface-Enhanced Raman Scattering in Au/ZnO/PATP Assembly. J. Phys. Chem. C 2009, 113, 117–120. [Google Scholar] [CrossRef]
- Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Graphene based nanoassembly for simultaneous detection and degradation of harmful organic contaminants from aqueous solution. RCS Adv. 2016, 6, 34342–34349. [Google Scholar] [CrossRef]
- Ko, Y.Y.; Fang, H.Y.; Chen, D.H. Fabrication of Ag/ZnO/reduced graphene oxide nanocomposite for SERS detection and multiway killing of bacteria. J. Alloys Compd. 2017, 695, 1145–1153. [Google Scholar] [CrossRef]
- Barbillon, G.; Sandana, V.E.; Humbert, C.; Belier, B.; Rogers, D.J.; Teherani, F.H.; Bove, P.; McClintock, R.; Razeghi, M. Study of Au coated ZnO nanoarrays for surface enhanced Raman scattering chemical sensing. J. Mater. Chem. C 2017, 5, 3528–3535. [Google Scholar] [CrossRef]
- Sivapalan, S.T.; DeVetter, B.M.; Yang, T.K.; van Dijk, T.; Schulmerich, M.V.; Carney, P.S.; Bhargava, R.; Murphy, C.J. Off-Resonance Surface-Enhanced Raman Spectroscopy from Gold Nanorod Suspensions as a Function of Aspect Ratio: Not What We Thought. ACS Nano 2013, 7, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, J.D.; Vijayaragavan, V.; Bhakoo, K.K.; Tan, T.T.Y. Tuning sub-10 nm single-phase NaMnF3 nanocrystals as ultrasensitive hosts for pure intense fluorescence and excellent T1 magnetic resonance imaging. Chem. Commun. 2012, 48, 10322. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.A. Surface raman electromagnetic enhancement factors for molecules at the surface of small isolated metal spheres: The determination of adsorbate orientation from sers relative intensities. Surf. Sci. 1983, 124, 209–219. [Google Scholar] [CrossRef]
- Moskovits, M.; Suh, J.S. Surface Selection Rules for Surface-Enhanced Raman Spectroscopy: Calculations and Application to the Surface-Enhanced Raman Spectrum of Phthalazine on Silver J. Phy. Chem. 1984, 88, 5526–5530. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can Graphene be used as a Substrate for Raman Enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, L.; Jiang, T.; Bai, Z.; Yu, X.; Dai, P.; Wu, M. Controllable SERS performance for the flexible paper-like films of reduced graphene oxide. Appl. Surf. Sci. 2017, 419, 373–381. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Wang, M.; Zhang, L.; Chu, B.; Yang, C.; Liu, Y.; Zhou, D.; Lu, Y. Constructing sub-10-nm gaps in graphene-metal hybrid system for advanced surface-enhanced Raman scattering detection. J. Alloys Compd. 2017, 720, 139–146. [Google Scholar] [CrossRef]
- Tan, C.; Huang, X.; Zhang, H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today 2013, 16, 29–36. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, C.H. Graphene thickness-controlled photocatalysis and surface enhanced Raman scattering. Nanoscale 2014, 6, 12805–12813. [Google Scholar] [CrossRef] [PubMed]













| Substrates | Morphology | Probes | EF/Detection Limits | Mechanism | References |
|---|---|---|---|---|---|
| ZnO | Colloids | D266 | More than 50 | CM | [30] |
| ZnO | Nanocrystals | 4-Mpy | 103 | CM | [72] |
| ZnO | Nanoparticles | 4-MBA & 4-Mpy | - | CM | [85] |
| ZnO | Nanowires, nanocones | 4-Mpy | 103 | “Hot spots” + cavity-like structural resonance | [111] |
| ZnO | Porous nanosheets | 4-MBA | 103/10−6M | CM | [94] |
| ZnO | ZnO nanorod arrays sheathed with ZnO nanocrystals | 4-Mpy | 68 | CM | [112] |
| a-ZnO | Nanocages | 4-Mpy | 6.62 × 105 | CM | [91] |
| Co-doping ZnO | Nanoparticles | 4-MBA | - | CM | [83] |
| Mg-doping ZnO | Nanoparticles | 4-MBA | - | CM | [106] |
| Ag/ZnO | Microspheres | 4-Mpy | 9 × 104 | EM + CM | [88] |
| Ag/ZnO | Wheatear-like ZnO nanoarrays decorated with Ag nanoparticles | R6G | 4.9 × 107 | EM + CM | [98] |
| Ag/ZnO | Worm-like Ag-coated ZnO nanowires | R6G | 3.082 × 107 /10−10M | EM + CM | [97] |
| Ag/ZnO | ZnO nanowires deposited on an Ag foil surface | PATP | 1.2 × 108 /10−12M | EM + CM | [113] |
| Ag/ZnO | Urchin-like Ag NPs deposited on ZnO hollow nanosphere arrays | R6G | 108/10−10M | CM + “hot spots” | [114] |
| Ag/ZnO | Ag-nanoparticle-decorated Si/ZnO nanotrees | R6G | 1 × 106 | EM + CM + structure- induced light trapping | [105] |
| Ag/ZnO | Ag nanoparticles deposited on ZnO nanowire arrays | Malachite green (MG)/amoxicillin | MG (2.5 × 1010 /10−12 M) Amoxicillin (10−9M) | “Hot spots” | [18] |
| Au/ZnO | Dendritic Au/ZnO composite | R6G | 10−9M | EM + CM | [106] |
| Au/ZnO | Au-coated ZnO nanowires | 4-methylbenzenethiol (4-MBT)/1,2-benzendithiol (1,2-BDT) | 2.19 × 106/ 4 × 105M | EM + CM + “hot spots” | [50] |
| Au/ZnO | Flower-shaped ZnO-nanopyramids-coated Au core | PATP | - | CM of ZnO greatly excited by LSPR of Au core | [74] |
| Au/ZnO | Au-coated ZnO nanorods | MB | 10−12M | “Hot spots” | [115] |
| Au/ZnO | Au nano-porous structure electroplated on ZnO nanorods | R6G | 2.24 × 106 | “Hot spots” | [116] |
| ZnO/Ag/Au NPs | Ultrasharp nanocones | Benzenethiol (BT), R6G, adenine | 1010–1011/BT (10−19 M), R6G (10−17 M), adenine (10−17 M) | EM + CM + “hot spots” | [99] |
| Au/ZnO/PATP | Layer-by-layer assembly | PATP | - | CM | [117] |
| ZnO-Ag- graphene nanosheets | Core–shell nanostructure integrated on nanosheets | Acridine orange (AO) dye | - | EM + CM | [118] |
| Ag/ZnO/rGO | Ag nanoparticles deposited on ZnO/rGO nanocomposite | E.coli | 104 cfu/mL | EM | [119] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yang, Y.; Ma, Y.; Li, S.; Wei, Y.; Huang, Z.; Long, N.V. Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application. Nanomaterials 2017, 7, 398. https://doi.org/10.3390/nano7110398
Yang L, Yang Y, Ma Y, Li S, Wei Y, Huang Z, Long NV. Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application. Nanomaterials. 2017; 7(11):398. https://doi.org/10.3390/nano7110398
Chicago/Turabian StyleYang, Lili, Yong Yang, Yunfeng Ma, Shuai Li, Yuquan Wei, Zhengren Huang, and Nguyen Viet Long. 2017. "Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application" Nanomaterials 7, no. 11: 398. https://doi.org/10.3390/nano7110398
APA StyleYang, L., Yang, Y., Ma, Y., Li, S., Wei, Y., Huang, Z., & Long, N. V. (2017). Fabrication of Semiconductor ZnO Nanostructures for Versatile SERS Application. Nanomaterials, 7(11), 398. https://doi.org/10.3390/nano7110398