A Facile Approach to Tune the Electrical and Thermal Properties of Graphene Aerogels by Including Bulk MoS2
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Graphene Aerogels
2.3. Characterization
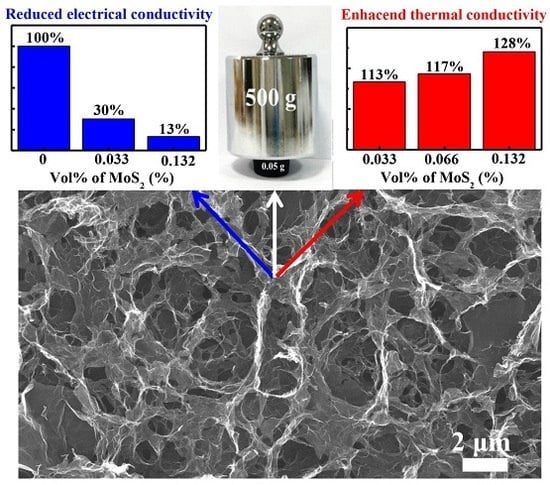
3. Results and Discussion
3.1. Macro-Scale Morphology and Mechanical Property of Graphene Aerogels
3.2. Micro-Scale Morphology of Pure GA and Composite GA (with MoS2)
3.3. Electrical Property of Pure GA and Composite GA
3.4. Thermal Transport Property of Pure GAs and Composite GAs
- (1)
- MoS2 reduced the pore size and lowered the surface area in composite GAs, thus decreasing the probability of phonon scattering at air-graphene interfaces.
- (2)
- The MoS2 may uniformly disperse at graphene-graphene interfaces to lower the interfacial thermal resistances, thus accelerating the transfer of heat among graphene.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xia, F.; Farmer, D.B.; Lin, Y.-M.; Avouris, P. Graphene Field-Effect Transistors with High On/Off Current Ratio and Large Transport Band Gap at Room Temperature. Nano Lett. 2010, 10, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Sfeir, M.Y.; Wu, Y.; Lui, C.H.; Misewich, J.A.; Heinz, T.F. Measurement of the Optical Conductivity of Graphene. Phys. Rev. Lett. 2008, 101, 196405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Ismail, I.M.; Stein, A. Ultralight, high-surface-area, multifunctional graphene-based aerogels from self-assembly of graphene oxide and resol. Carbon 2014, 68, 221–231. [Google Scholar] [CrossRef]
- Guo, K.; Hu, Z.; Song, H.; Du, X.; Zhong, L.; Chen, X. Low-density graphene/carbon composite aerogels prepared at ambient pressure with high mechanical strength and low thermal conductivity. RSC Adv. 2015, 5, 5197–5204. [Google Scholar] [CrossRef]
- Sui, Z.; Meng, Q.; Zhang, X.; Ma, R.; Cao, B. Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification. J. Mater. Chem. 2012, 22, 8767–8771. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Müllen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, B.; Li, X.; Song, X.; Xia, H.; Li, L.; Zeng, H. Monolayer MoS2–Graphene Hybrid Aerogels with Controllable Porosity for Lithium-Ion Batteries with High Reversible Capacity. ACS Appl. Mater. Interfaces 2016, 8, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Samad, Y.A.; Li, Y.; Schiffer, A.; Alhassan, S.M.; Liao, K. Graphene Foam Developed with a Novel Two-Step Technique for Low and High Strains and Pressure-Sensing Applications. Small 2015, 11, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat Nano 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, S.; Xu, Z.; Wu, H.; Deng, C.; Wang, X. Interface-mediated extremely low thermal conductivity of graphene aerogel. Carbon 2016, 98, 381–390. [Google Scholar] [CrossRef]
- Worsley, M.A.; Shin, S.J.; Merrill, M.D.; Lenhardt, J.; Nelson, A.J.; Woo, L.Y.; Gash, A.E.; Baumann, T.F.; Orme, C.A. Ultralow Density, Monolithic WS2, MoS2, and MoS2/Graphene Aerogels. ACS Nano 2015, 9, 4698–4705. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lv, Y.; Gong, F.; Elzatahry, A.A.; Zheng, G.; Zhao, D. Synthesis of 2D-Mesoporous-Carbon/MoS2 Heterostructures with Well-Defined Interfaces for High-Performance Lithium-Ion Batteries. Adv. Mater. 2016, 28, 9385–9390. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, K.; Fu, H.; Guo, B.; Lei, X.; Zhu, Z. Multi-slice nanostructured WS2@rGO with enhanced Li-ion battery performance and a comprehensive mechanistic investigation. Phys. Chem. Chem. Phys. 2015, 17, 29824–29833. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tng, D.Z.Y.; Nguyen, S.T.; Feng, J.D.; Lin, C.F.; Xiao, P.F.; Lu, L.; Duong, H.M. Morphology effects on electrical and thermal properties of binderless graphene aerogels. Chem. Phys. Lett. 2013, 561, 92–96. [Google Scholar] [CrossRef]
- Menzel, R.; Barg, S.; Miranda, M.; Anthony, D.B.; Bawaked, S.M.; Mokhtar, M.; Al-Thabaiti, S.A.; Basahel, S.N.; Saiz, E.; Shaffer, M.S.P. Joule Heating Characteristics of Emulsion-Templated Graphene Aerogels. Adv. Funct. Mater. 2015, 25, 28–35. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced mechanical, thermal, and electric properties of graphene aerogels via supercritical ethanol drying and high-temperature thermal reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tng, D.Z.Y.; Lim, C.X.T.; Liu, P.; Nguyen, S.T.; Xiao, P.; Marconnet, A.; Lim, C.Y.H.; Duong, H.M. Thermal and electrical properties of graphene/carbon nanotube aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 48–53. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Kondo, H.; Hori, M. Graphene Nanowalls. In New Progress on Graphene Research; Gong, J.R., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Zhang, L.; Chen, G.; Hedhili, M.N.; Zhang, H.; Wang, P. Three-dimensional assemblies of graphene prepared by a novel chemical reduction-induced self-assembly method. Nanoscale 2012, 4, 7038–7045. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Harle, A.; Sathaye, S.; Patil, K. Development of a novel method to grow mono-/few-layered MoS2 films and MoS2-graphene hybrid films for supercapacitor applications. CrystEngComm 2014, 16, 10845–10855. [Google Scholar] [CrossRef]
- El-Mahalawy, S.H.; Evans, B.L. Temperature Dependence of the Electrical Conductivity and Hall Coefficient in 2H-MoS2, MoSe2, WSe2, and MoTe2. Phys. Status Solidi B Basic Res. 1977, 79, 713–722. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Yan, R.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.; Kis, A.; Luo, T.; Hight Walker, A.R.; Xing, H.G. Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Naidu, G.A.; Udo, S. Thermal conductivity of bulk and monolayer MoS2. EPL (Europhys. Lett.) 2016, 113, 36002. [Google Scholar]
- Liu, J.; Choi, G.-M.; Cahill, D.G. Measurement of the anisotropic thermal conductivity of molybdenum disulfide by the time-resolved magneto-optic Kerr effect. J. Appl. Phys. 2014, 116, 233107. [Google Scholar] [CrossRef]
- Sahoo, S.; Gaur, A.P.S.; Ahmadi, M.; Guinel, M.J.F.; Katiyar, R.S. Temperature-Dependent Raman Studies and Thermal Conductivity of Few-Layer MoS2. J. Phys. Chem. C 2013, 117, 9042–9047. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Shen, S.; Zhuang, J.; Wang, X. Noble-Metal-Promoted Three-Dimensional Macroassembly of Single-Layered Graphene Oxide. Angew. Chem. 2010, 122, 4707–4711. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene-Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Bui, K.; Papavassiliou, D.V.; Duong, H.M. Thermal transport phenomena and limitations in heterogeneous polymer composites containing carbon nanotubes and inorganic nanoparticles. Carbon 2014, 78, 305–316. [Google Scholar] [CrossRef]
- Gong, F.; Duong, H.M.; Papavassiliou, D.V. Inter-Carbon Nanotube Contact and Thermal Resistances in Heat Transport of Three-Phase Composites. J. Phys. Chem. C 2015, 119, 7614–7620. [Google Scholar] [CrossRef]
- Fan, Z.; Gong, F.; Nguyen, S.T.; Duong, H.M. Advanced multifunctional graphene aerogel—Poly(methyl methacrylate) composites: Experiments and modeling. Carbon 2015, 81, 396–404. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.; Li, Y.; Lee, G.-H.; Cui, X.; Chenet, D.; You, Y.; Heinz, T.F.; Hone, J.C. Measurement of Lateral and Interfacial Thermal Conductivity of Single- and Bilayer MoS2 and MoSe2 Using Refined Optothermal Raman Technique. ACS Appl. Mater. Interfaces 2015, 7, 25923–25929. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Li, C.; Xu, S.; Liu, J.; Wang, X. Interfacial thermal conductance between few to tens of layered-MoS2 and c-Si: Effect of MoS2 thickness. Acta Mater. 2017, 122, 152–165. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Thermal conductivity of pure GA (W/mK) | 0.038 |
| Thermal conductivity of MoS2 (W/mK) [34,35,36,37] | 15 |
| Volume fraction of MoS2 f (%) | 0–0.5 |
| Thickness of the multilayered MoS2 (nm) | 10 |
| Interfacial thermal resistance (10−8 m2K/W) | 0.9 |
| Density of graphene (g/cm3) [38] | 1.06 |
| Density of MoS2 (g/cm3) | 4.8 |
| Density of air (g/cm3) | 0.0012 |
| Volume fraction of MoS2 (%) | Electrical conductivity (S/m) |
| 0 | 13.6 |
| 0.033 | 4.1 |
| 0.132 | 1.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, F.; Liu, X.; Yang, Y.; Xia, D.; Wang, W.; Duong, H.M.; Papavassiliou, D.V.; Xu, Z.; Liao, J.; Wu, M. A Facile Approach to Tune the Electrical and Thermal Properties of Graphene Aerogels by Including Bulk MoS2. Nanomaterials 2017, 7, 420. https://doi.org/10.3390/nano7120420
Gong F, Liu X, Yang Y, Xia D, Wang W, Duong HM, Papavassiliou DV, Xu Z, Liao J, Wu M. A Facile Approach to Tune the Electrical and Thermal Properties of Graphene Aerogels by Including Bulk MoS2. Nanomaterials. 2017; 7(12):420. https://doi.org/10.3390/nano7120420
Chicago/Turabian StyleGong, Feng, Xiongxiong Liu, Yunlong Yang, Dawei Xia, Wenbin Wang, Hai M. Duong, Dimitrios V. Papavassiliou, Ziqiang Xu, Jiaxuan Liao, and Mengqiang Wu. 2017. "A Facile Approach to Tune the Electrical and Thermal Properties of Graphene Aerogels by Including Bulk MoS2" Nanomaterials 7, no. 12: 420. https://doi.org/10.3390/nano7120420
APA StyleGong, F., Liu, X., Yang, Y., Xia, D., Wang, W., Duong, H. M., Papavassiliou, D. V., Xu, Z., Liao, J., & Wu, M. (2017). A Facile Approach to Tune the Electrical and Thermal Properties of Graphene Aerogels by Including Bulk MoS2. Nanomaterials, 7(12), 420. https://doi.org/10.3390/nano7120420