Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment
Abstract
:1. Introduction
2. Experiment
2.1. Chemical Reagents
2.2. Synthesis of HAp Nanoparticles
2.3. Synthesis of Magnetic Fe3O4 Nanoparticles
2.4. Synthesis of Fe3O4-HAp (IO-HAp) Nanocomposites
2.5. Characterization
2.6. Cell Culture
2.7. Cell Viability Assay
2.7.1. MTT Assay
2.7.2. Trypan Blue Study
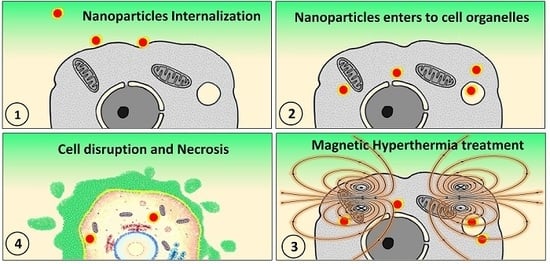
2.8. Nanoparticles Internalization Study by Cells
2.9. Magnetic Hyperthermia Experiment
2.10. In Vitro Magnetic Hyperthermia
3. Results and Discussion
3.1. Morphological Characterizations of the Materials
3.1.1. X-ray Diffraction (XRD) Analysis
3.1.2. Fourier Transform Infrared Spectroscopy
3.1.3. Thermogravimetric Analysis
3.1.4. FETEM, DLS and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
3.1.5. Vibrating Sample Magnetometer
3.1.6. Cytotoxicity Testing
3.1.7. Trypan Blue Study
3.1.8. Nanoparticles Internalization Study by Cells
3.2. Magnetic Hyperthermia Ability
3.2.1. Magnetic Hyperthermia on MG-63 Cells
3.2.2. Measurement of SAR Effect
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corchero, J.L.; Villaverde, A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009, 27, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Drug Targeting. Eur. J. Pharm. Sci. 2000, 11, S81–S91. [Google Scholar] [CrossRef]
- Bretcanu, O.; Miola, M.; Bianchi, C.L.; Marangi, I.; Carbone, R.; Corazzari, I.; Cannas, M.; Verné, E. In vitro biocompatibility of a ferrimagnetic glass-ceramic for hyperthermia application. Mater. Sci. Eng. C 2017, 73, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, G.H. Physical Aspects of Hyperthermia; American Institute of Physics: College Park, MD, USA, 1982; p. 656. ISBN 9780883184141. [Google Scholar]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cervadoro, A.; Ramirez, M.R.; Stigliano, C.; Brazdeikis, A.; Colvin, V.L.; Civera, P.; Key, J.; Decuzzi, P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrot, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Fruhauf, K.; Andra, W.; Hiergeist, R.; Hergt, R.; Kaiser, W.A. Heating potential of iron oxides for therapeutic purposes in interventional radiology. Acad. Radiol. 2002, 9, 198–202. [Google Scholar] [CrossRef]
- Hilger, I.; Andra, W.; Hergt, R.; Hiergeist, R.; Schubert, H.; Kaiser, W.A. Electromagnetic heating of breast tumours in interventional radiology: In vitro and in vivo studies in human cadavers and mice. Radiology 2001, 218, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Scholz, R.; Tesche, B.; Fahling, H.; Mitrovics, T.; Vogl, T.; Cervos-Navarro, J.; Felix, R. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int. J. Hyperth. 1996, 12, 705–722. [Google Scholar] [CrossRef]
- Lubbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, R.; Philippe, P.; Sophie, P.; Abderrahim, N.; Cécile, R.; Claire, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: Mechanisms and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar]
- Moreno, E.M.; Zayat, M.; Morales, M.P.; Serna, C.J.; Roig, A.; Levy, D. Preparation of narrow size distribution superparamagnetic c-Fe2O3 nanoparticles in a sol-gel transparent SiO2 matrix. Langmuir 2002, 18, 4972–4978. [Google Scholar] [CrossRef]
- Ansar, E.B.; Ajeesh, M.; Yokogawa, Y.; Wunderlich, W.; Varma, H. Synthesis and characterization of iron oxide embedded hydroxyapatite bioceramics. J. Am. Ceram. Soc. 2012, 95, 2695–2699. [Google Scholar] [CrossRef]
- Rogers, W.J.; Basu, P. Factors regulating macrophage endocytosis of nanoparticles: Implications for targeted magnetic resonance plaque imaging. Atherosclerosis 2005, 178, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh, M.; Francis, B.F.; Annie, J.; Varma, P.R.H. Nano iron oxide-hydroxyapatite composite ceramics with enhanced radiopacity. J. Mater. Sci. Mater. Med. 2010, 21, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.S.; Elayaraja, K.; Arul, K.T.; Ferraris, S.; Spriano, S.; Ferraris, M.; Asokan, K.; Kalkura, S.N. Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 2015, 41, 13153–13163. [Google Scholar] [CrossRef]
- Ramya, J.R.; Arul, K.T.; Elayaraja, K.; Kalkura, S.N. Physicochemical and biological properties of iron and zinc ions co-doped nanocrystalline hydroxyapatite, synthesized by ultrasonication. Ceram. Int. 2014, 40, 16707–16717. [Google Scholar] [CrossRef]
- Murakami, S.; Hosono, T.; Jeyadevan, B.; Kamitakahara, M.; Ioku, K. Hydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancer. J. Am. Ceram. Soc. 2008, 116, 950–954. [Google Scholar] [CrossRef]
- Donadel, K.; Felisberto, M.D.V.; Laranjei, M.C.M. Preparation and characterization of hydroxyapatite-coated iron oxide particles by spray-drying technique. Anais da Academia Brasileira de Ciências 2009, 81, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.H.; Hou, S.M.; Hsueh, Y.S.; Lin, J.; Wu, H.C.; Lin, F.H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U.; Dey, A. Natural origin hydroxyapatite scaffold as potential bone tissue engneering substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Madrid, S.I.U.; Pal, U.; Jesús, F.S. Controlling size and magnetic properties of Fe3O4 clusters in solvothermal process. Adv. Nano Res. 2014, 2, 187–198. [Google Scholar] [CrossRef]
- Mondal, S.; Mahata, S.; Kundu, S.; Mondal, B. Processing of natural resourced hydroxyapatite ceramics from fish scale. Adv. Appl. Ceram. 2010, 109, 234–239. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, B.; Dey, A.; Mukhopadhyay, S.S. Studies on processing and characterization of hydroxyapatite biomaterials from different bio wastes. J. Miner. Mater. Charact. Eng. 2012, 11, 55–67. [Google Scholar] [CrossRef]
- Zakharov, N.A.; Polunina, I.A.; Polunin, K.E.; Rakitina, N.M.; Kochetkova, E.I.; Sokolova, N.P.; Kalinnikov, V.T. Calcium hydroxyapatite for medical applications. Inorg. Mater. 2004, 40, 641–648. [Google Scholar] [CrossRef]
- Sneha, M.; Sundaram, M.N. Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int. J. Nanomed. 2015, 10, 99–106. [Google Scholar]
- Willis, A.L.; Turro, N.J.; O’Brien, S. Spectroscopic characterization of the surface of iron oxide nanocrystals. Chem. Mater. 2005, 17, 5970–5975. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yasukawa, A.; Kandori, K.; Ishikawa, T. FTIR Study on incorporation of into calcium hydroxyapatite. J. Chem. Soc. Faraday Trans. 1998, 94, 1501–1505. [Google Scholar] [CrossRef]
- Wu, H.S.; Wang, T.W.; Sun, J.S.; Wang, W.H.; Lin, F.H. A novel biomagnetic nanoparticle based on hydroxyapatite. Nanotechnology 2007, 18, 165601. [Google Scholar] [CrossRef]
- Gambardella, A.; Bianchi, M.; Kaciulis, S.; Mezzi, A.; Brucale, M.; Cavallini, M.; Herrmannsdoerfer, T.; Chanda, G.; Uhlarz, M.; Cellini, A.; et al. Magnetic hydroxyapatite coatings as a new tool in medicine: A scanning probe investigation. Mater. Sci. Eng. C 2016, 62, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011, 7, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Dong, L.; Lu, Y.; Zhang, L.C.; An, D.; Gao, H.L.; Yang, D.M.; Hu, W.; Sui, C.; Xu, W.P.; et al. Magnetic hydroxyapatite nanoworms for magnetic resonance diagnosis of acute hepatic injury. Nanoscale 2016, 8, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Prabhu, D.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Facile in situ growth of Fe3O4 nanoparticles on hydroxyapatite nanorods for pH dependent adsorption and controlled release of proteins. RSC Adv. 2014, 4, 50510–50520. [Google Scholar] [CrossRef]
- Chen, F.; Li, C.; Zhu, Y.J.; Zhao, X.Y.; Lu, B.Q.; Wu, J. Magnetic nanocomposite of hydroxyapatite ultrathin nanosheets/Fe3O4 nanoparticles: Microwave-assisted rapid synthesis and application in pH-responsive drug release. Biomater. Sci. 2013, 1, 1074–1081. [Google Scholar] [CrossRef]
- Singh, R.K.; El-Fiqi, A.M.; Patel, K.D.; Kim, H.W. A novel preparation of magnetic hydroxyapatite nanotubes. Mater. Lett. 2012, 75, 130–133. [Google Scholar] [CrossRef]
- Gopi, D.; Ansari, M.T.; Shinyjoy, E.; Kavitha, L. Synthesis and spectroscopic characterization of magnetic hydroxyapatite nanocomposite using ultrasonic irradiation. Spectrochim. Acta Part A 2012, 87, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.T.; Wang, H.; Li, L.; Liu, Y.S.; Deng, X.B.; Huo, S.F.; Yuan, F.F.; Liu, Z.F.; Tong, H.S.; Su, L. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci. Rep. 2014, 4, 4469. [Google Scholar] [CrossRef] [PubMed]
- Skibba, J.L.; Powers, R.H.; Stadnicka, A.; Cullinane, D.W.; Almagro, U.A.; Kalbfleisch, J.H. Oxidative stress as a precursor to the irreversible hepatocellular injury caused by hyperthermia. Int. J. Hyperth. 1991, 7, 749–761. [Google Scholar] [CrossRef]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 2017, 113, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Oh, Y.; Bharathiraja, S.; Manivasagan, P.; Rajarathinam, T.; Jang, B.; Vy Phan, T.T.; Jang, H.; Oh, J. Synthesis of amine-polyglycidol functionalized Fe3O4@SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and bioimaging applications. RSC Adv. 2017, 6, 110444–110453. [Google Scholar] [CrossRef]









| Sl. No. | Wavenumber cm−1 | Functional Groups | Reference | ||
|---|---|---|---|---|---|
| HAp | IO | IO-HAp | |||
| 1 | 3450–3575 | 3450–3575 | 3450–3575 | O–H group stretching vibration | [25,26] |
| 2 | 462–479 | 479 | 462 | ν2 bending vibration of P–O | [27] |
| 3 | 1646 | -- | 1641 | O–H adsorbed water | [30] |
| 4 | 1452 | -- | 1448 | C–O stretching vibration | [30] |
| 5 | -- | 1060 | -- | weak vibration of Fe–OH group | [28] |
| 6 | 1098–1046 | -- | 1046 | n3 vibration of P–O | [25,26] |
| 7 | 883 | -- | 883 | v2 bending C–O | [30] |
| 8 | 634 | -- | 634 | libration mode of the O–H | [25,26] |
| 9 | 605 | -- | 605 | n4 bending mode of P–O | [25,26] |
| 10 | 567 | -- | 567 | ν4 bending of P–O group | [25,26] |
| 11 | -- | 570 | -- | Fe–O vibrations for IO | [25,28] |
| 12 | -- | 2920 | 2920 | C–H vibration | [29] |
| Sl. No. | Synthesis Route | Application | Saturation Magnetization (Ms) | Reference |
|---|---|---|---|---|
| 1 | Wet precipitation technique | Biomedicine application, Hyperthermia study. | 7.23–20.92 emu/g | [22,31] |
| 2 | Pulsed plasma deposition | Biofilm formation | 0.26 emu/g | [32] |
| 3 | Hydrothermal method | Biomedicine applications | ~0.32 emu/g | [33] |
| 4 | Spray-drying technique | Medical diagnosis and imaging | ~12 emu/g | [20] |
| 5 | Microwave route | pH-responsive drug release | 18.9 emu/g | [34] |
| 6 | Multi step synthesis: wet precipitation, hydrothermal, ultrasonication, and layer by layer coating | Magnetic resonance imaging, Drug delivery | ~4–7 emu/g | [35] |
| 7 | Hydrothermal method | pH dependent protein adsorption release carrier | 11.5–15.5 emu/g | [36] |
| 8 | Polymer templated electrospun technique | Biomedical and hyperthermia treatment | 27.20 emu/g | [37] |
| 9 | Ultrasonic irradiation technique | Biomedical | 7.40 emu/g | [38] |
| 10 | Chemical precipitation | Magnetic hyperthermia for cancer treatment | 40.6 emu/g | Present Study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials 2017, 7, 426. https://doi.org/10.3390/nano7120426
Mondal S, Manivasagan P, Bharathiraja S, Santha Moorthy M, Nguyen VT, Kim HH, Nam SY, Lee KD, Oh J. Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials. 2017; 7(12):426. https://doi.org/10.3390/nano7120426
Chicago/Turabian StyleMondal, Sudip, Panchanathan Manivasagan, Subramaniyan Bharathiraja, Madhappan Santha Moorthy, Van Tu Nguyen, Hye Hyun Kim, Seung Yun Nam, Kang Dae Lee, and Junghwan Oh. 2017. "Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment" Nanomaterials 7, no. 12: 426. https://doi.org/10.3390/nano7120426
APA StyleMondal, S., Manivasagan, P., Bharathiraja, S., Santha Moorthy, M., Nguyen, V. T., Kim, H. H., Nam, S. Y., Lee, K. D., & Oh, J. (2017). Hydroxyapatite Coated Iron Oxide Nanoparticles: A Promising Nanomaterial for Magnetic Hyperthermia Cancer Treatment. Nanomaterials, 7(12), 426. https://doi.org/10.3390/nano7120426