Novel Mesoporous Flowerlike Iron Sulfide Hierarchitectures: Facile Synthesis and Fast Lithium Storage Capability
Abstract
:1. Introduction
2. Materials and Methods Results
2.1. Synthesis of 3D Flowerlike Fe-Based Precursor
2.2. Synthesis of Uniform 3D F-FeS Nanostructure
2.3. Materials Characterization
2.4. Electrochemical Measurements
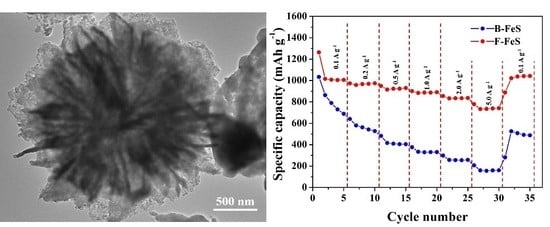
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Tavassol, H.; Jones, E.M.; Sottos, N.R.; Gewirth, A.A. Electrochemical stiffness in lithium-ion batteries. Nat. Mater. 2016, 15, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Luo, B.; Jia, Y.; Zhi, L. One-dimensional/two-dimensional hybridization for self-supported binder-free silicon-based lithium ion battery anodes. Nanoscale 2013, 5, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gu, L.; Wang, C.; Dhanabalan, A.; Van Aken, P.; Maier, J. Encapsulation of Sn@carbon nanoparticles in bamboo-like hollow carbon nanofibers as an anode material in lithium-based batteries. Angew. Chem. Int. Ed. 2009, 48, 6485–6489. [Google Scholar] [CrossRef] [PubMed]
- Ricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar]
- Guo, Y.G.; Hu, J.S.; Wan, L.J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Azami, R.; Reynier, Y.F. Mechanism of self-discharge in graphite–lithium anode. Electrochim. Acta 2003, 47, 1217–1223. [Google Scholar] [CrossRef]
- Nazar, L.F.; Goward, G.; Leroux, F.; Duncan, M.; Huang, H.; Kerr, T. Nanostructured materials for nergy storage. Int. J. Inorg. Mater. 2001, 3, 191–200. [Google Scholar] [CrossRef]
- Irscher, M. Nanoscale materials for energy storage. Mater. Sci. Eng. B 2004, 108, 1. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Hu, Y.S.; Kienle, L.; Guo, Y.G.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 37, 1421–1426. [Google Scholar] [CrossRef]
- Penki, T.R.; Shivakumara, S.; Minakshi, M.; Munichandraiah, N. Porous flower-like alpha-Fe2O3 nanostructure: A high performance anode material for lithium-ion batteries. Electrochim. Acta 2015, 167, 330–339. [Google Scholar] [CrossRef]
- Gou, J.; Xie, S.; Liu, Y.; Liu, C. Flower-like nickel-cobalt hydroxides converted from phosphites for high rate performance hybrid supercapacitor electrode materials. Electrochim. Acta 2016, 210, 915–924. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. L-cysteine-assisted synthesis of layered MoS2/graphene composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 5, 4720–4728. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, Y.; Wang, Y. Graphene-wrapped cos nanoparticles for high-capacity lithium-ion storage. ACS Appl. Mater. Interfaces 2013, 5, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Lou, X.W. Formation of CoS2 nanobubble hollow prisms for highly reversible lithium storage. Angew. Chem. Int. Ed. 2016, 128, 13422–13426. [Google Scholar] [CrossRef] [PubMed]
- Bing, Z.; Wang, Z.; Fang, C.; Yang, Y.; Yang, G.; Lu, C. Three-dimensional interconnected spherical graphene framework/sns nanocomposite for anode material with superior lithium storage performance: Complete reversibility of Li2S. ACS Appl. Mater. Interfaces 2017, 9, 1407–1415. [Google Scholar]
- Guan, D.; Ma, L.; Pan, D.; Li, J.; Gao, X.; Xie, Y. Atomic layer deposition of alumina coatings onto SnS2 for lithium-ion battery applications. Electrochim. Acta 2017, 242, 117–124. [Google Scholar] [CrossRef]
- Wang, J.; Bai, F.; Chen, X.; Lu, Y.; Yang, W. Intercalated Co(OH)2-derived flower-like hybrids composed of cobalt sulfide nanoparticles partially embedded in nitrogen-doped carbon nanosheets with superior lithium storage. J. Mater. Chem. A 2017, 5, 3628–3637. [Google Scholar] [CrossRef]
- Hu, S.; Chen, W.; Zhou, J.; Yin, F.; Uchaker, E.; Zhang, Q. Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 7862–7872. [Google Scholar] [CrossRef]
- Li, L.; Cheah, Y.; Ko, Y.; Teh, P.; Wee, G.; Wong, C. The facile synthesis of hierarchical porous flower-like NiCo2O4 with superior lithium storage properties. J. Mater. Chem. A 2013, 1, 10935–10941. [Google Scholar] [CrossRef]
- Zhu, C.; Wen, Y.; Van Aken, P.A.; Maier, J.; Yu, Y. High lithium storage performance of FeS nanodots in porous graphitic carbon nanowires. Adv. Funct. Mater. 2015, 25, 2335–2342. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Zhou, J.; Chen, X. Iron sulfide-embedded carbon microsphere anode material with high-rate performance for lithium-ion batteries. Chem. Commun. 2011, 47, 8653–8655. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.S.; Kang, Y.C. Porous FeS nanofibers with numerous nanovoids obtained by Kirkendall diffusion effect for use as anode materials for sodium-ion batteries. Nano Res. 2017, 10, 897–907. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, Y.C. Sodium-Ion Storage Properties of FeS-Reduced Graphene Oxide Composite Powder with a Crumpled Structure. Chem. Eur. J. 2016, 22, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hwang, J.Y.; Belharouak, I.; Sun, Y.K. Na-storage capability investigation of carbon nanotubes-encapsulated Fe1−xS composite. ACS Energy. Lett. 2017, 2, 364–372. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Y.; Rui, X.; Xiao, N.; Zhu, J.; Zhang, W. Controlled soft-template synthesis of ultrathin C@FeS nanosheets with high-li-storage performance. ACS Nano 2012, 6, 4713–4721. [Google Scholar]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.S. In-situ incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Li, L.; Raji, A.R.; Tour, J.M. Graphene-wrapped MnO2 -graphene nanoribbons as anode materials for high-performance lithium ion batteries. Adv. Mater. 2013, 25, 6298. [Google Scholar] [CrossRef] [PubMed]
- Fong, R.; Dahn, J.R.; Jones, C.H.W. Electrochemistry of pyrite-based cathodes for ambient temperature lithium batteries. J. Electrochem. Soc. 1989, 136, 3206–3210. [Google Scholar] [CrossRef]
- Fong, R.; Jones, C.H.W.; Dahn, J.R. A study of pyrite-based cathodes for ambient temperature lithium batteries by in situ 57 Fe mössbauer spectroscopy. J. Power Sources 1989, 26, 333–339. [Google Scholar] [CrossRef]
- Kim, B.C.; Takada, K.; Ohta, N.; Seino, Y.; Zhang, L.; Wada, H. All solid state li-ion secondary battery with FeS anode. Sol. State Ion. 2005, 176, 2383–2387. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Q.; Wang, C.; Zachariah, M.R. Interdispersed amorphous MnOx–carbon nanocomposites with superior electrochemical performance as lithium-storage material. Adv. Funct. Mater. 2012, 2, 803–811. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Ivanovici, S.; Müllen, K. Fabrication of graphene-encapsulated oxide nanoparticles: Towards high-performance anode materials for lithium storage. Angew. Chem. Int. Ed. 2010, 49, 8408–8411. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xi, K.; Tan, G.; Chen, S.; Zhao, T.; Coxon, P.R.; Kim, H.-K.; Ding, S.; Yang, Y.; Kumar, R.V.; et al. Sea urchin-like NiCoO2@C nanocomposites for Li-ion batteries and supercapacitors. Nano Energy 2016, 27, 457–465. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, K.; Zhu, Z.; Tao, Z.; Chen, J. FeS2 microspheres with an ether-based electrolyte for high-performance rechargeable lithium batteries. J. Mater. Chem. A 2015, 3, 12898–12904. [Google Scholar] [CrossRef]
- Wang, C.; Lan, M.; Zhang, Y.; Bian, H.; Yuen, M.F.; Ostrikov, K. Fe1−xS/C nanocomposites from sugarcane waste-derived microporous carbon for high-performance lithium ion batteries. Green Chem. 2016, 18, 3029–3039. [Google Scholar] [CrossRef]
- Xu, J.; Xue, H.; Yang, X.; Wei, H.; Li, W.; Li, Z. Synthesis of honeycomb-like mesoporous pyrite FeS2 microspheres as efficient counter electrode in quantum dots sensitized solar cells. Small 2014, 10, 4754–4759. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, X.; Zhi, L.; Cao, Q.; Maier, J.; Müllen, K. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage. Adv. Mater. 2010, 22, 838–842. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Zhuang, Q.; Liang, J.; Zhang, Z.; Liu, J.; Peng, H.; Mao, C.; Li, G. Novel Mesoporous Flowerlike Iron Sulfide Hierarchitectures: Facile Synthesis and Fast Lithium Storage Capability. Nanomaterials 2017, 7, 431. https://doi.org/10.3390/nano7120431
Ma Q, Zhuang Q, Liang J, Zhang Z, Liu J, Peng H, Mao C, Li G. Novel Mesoporous Flowerlike Iron Sulfide Hierarchitectures: Facile Synthesis and Fast Lithium Storage Capability. Nanomaterials. 2017; 7(12):431. https://doi.org/10.3390/nano7120431
Chicago/Turabian StyleMa, Quanning, Qianyu Zhuang, Jun Liang, Zhonghua Zhang, Jing Liu, Hongrui Peng, Changming Mao, and Guicun Li. 2017. "Novel Mesoporous Flowerlike Iron Sulfide Hierarchitectures: Facile Synthesis and Fast Lithium Storage Capability" Nanomaterials 7, no. 12: 431. https://doi.org/10.3390/nano7120431
APA StyleMa, Q., Zhuang, Q., Liang, J., Zhang, Z., Liu, J., Peng, H., Mao, C., & Li, G. (2017). Novel Mesoporous Flowerlike Iron Sulfide Hierarchitectures: Facile Synthesis and Fast Lithium Storage Capability. Nanomaterials, 7(12), 431. https://doi.org/10.3390/nano7120431