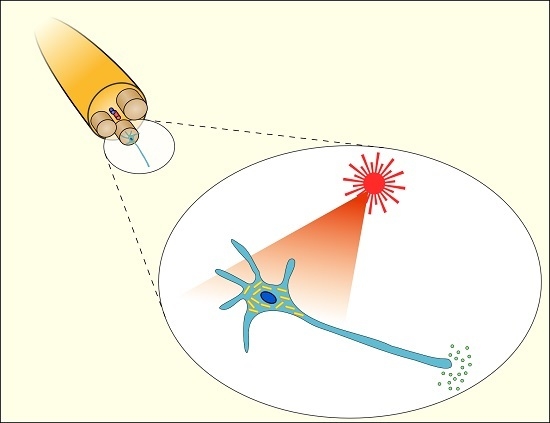
Gold Nanoparticles for Modulating Neuronal Behavior
Abstract
:1. Introduction
2. Properties of Gold Nanoparticles
2.1. Why Gold Nanoparticles?
2.2. Dynamics of the Localized Plasmon Resonance
3. Peripheral Nerve Regeneration
4. Modulation of Nerve Electrical Activity
5. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mclaughlin, S.; Podrebarac, J.; Ruel, M.; Suuronen, E.J.; McNeill, B.; Alarcon, E.I. Nano-engineered biomaterials for tissue regeneration: What has been achieved so far? Front. Mater. 2016, 3, 1–28. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Resting and action potentials in single nerve fibres. J. Physiol. Lond. 1945, 104, 176–195. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.H.A.; Haycock, J.W. Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Eng. B 2012, 18, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L. Nanomaterial-enabled neural stimulation. Front. Neurosci. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; Stoddart, P.R.; McArthur, S.L. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 2013, 110, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal delivery of polyethylene glycol-coated gold nanoparticles promotes functional recovery after spinal cord injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Haycock, J.W.; Cadusch, P.J.; McArthur, S.L.; Stoddart, P.R. Laser exposure of gold nanorods can induce intracellular calcium transients. J. Biophotonics 2014, 7, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Numata, T.; Morone, N.; Kaneko, S.; Mori, Y.; Imahori, H.; Murakami, T. Thermosensitive ion channel activation in single neuronal cells by using surface-engineered plasmonic nanoparticles. Angew. Chem. 2015, 54, 11725–11729. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Needham, K.; Brown, W.G.A.; Nayagam, B.A.; McArthur, S.L.; Yu, A.; Stoddart, P.R. Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv. Healthc. Mater. 2014, 3, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.; Kim, J.; Choi, J.M.; Kang, T.; Chang, J.W.; Byun, K.M.; Jun, S.B.; Kim, S.J. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 2014, 19, 3853–3857. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Hong, S.; Choi, Y.; Park, J.; Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 2014, 8, 8040–8049. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. J. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Carvalho-de-Souza, J.L.; Treger, J.S.; Dang, B.; Kent, S.B.H.; Pepperberg, D.R.; Bezanilla, F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 2015, 86, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Isomaa, B.; Reuter, J.; Djupsund, B.M. The subacute and chronic toxicity of cetyltrimethylammonium bromide (CTAB), a cationic surfactant, in the rat. Arch. Toxicol. 1976, 35, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Perez-Juste, J.; Liz-Marzan, L.M. Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chem. Mater. 2006, 18, 2465–2467. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, Y.G.; Jiang, L.P.; Zhu, J.J. Synthesis, characterizations of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin. Electrochem. Commun. 2008, 10, 355–358. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gu, M.M.; Zheng, T.T.; Zhu, J.J. Synthesis of gelatin-stabilized gold nanoparticles and assembly of carboxylic single-walled carbon nanotubes/Au composites for cytosensing and drug uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzan, L.M.; de Abajo, F.J.G. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Funston, A.M.; Novo, C.; Davis, T.J.; Mulvaney, P. Plasmon coupling of gold nanorods at short distances and in different geometries. Nano Lett. 2009, 9, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, G.; Costas, C.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Gold nanoparticles for regulation of cell function and behavior. Nano Today 2017, 13, 40–60. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Han, J.; Jang, H.K.; Noh, M.K.; La, W.G.; Yi, M.; Kim, W.S.; Kim Kwon, Y.; Yu, T.; Kim, B.S. Ph-triggered release of manganese from mnau nanoparticles that enables cellular neuronal differentiation without cellular toxicity. Biomaterials 2015, 55, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Sarma, M.G.; Borthakur, B.B.; Bora, U. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015, 62, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Jen, J.C.; Hsu, S.H.; Chiu, I.M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.; Hwang, S.; Yun, S.; Byun, K.M.; Jun, S.B.; Kim, S.J. Photothermal activation of astrocyte cells using localized surface plasmon resonance of gold nanorods. J. Biophotonics 2017, 10, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal analysis of gold nanorods heated with femtosecond laser pulses. J. Phys. D 2008, 41, 185501. [Google Scholar] [CrossRef] [PubMed]
- Plech, A.; Cerna, R.; Kotaidis, V.; Hudert, F.; Bartels, A.; Dekorsy, T. A surface phase transition of supported gold nanoparticles. Nano Lett. 2007, 7, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, W.; Skeini, T.; Richardson, H.; Lee, J.; Kotov, N.A. Gold nanoparticle ensembles as heaters and actuators: Melting and collective plasmon resonances. Nanoscale Res. Lett. 2006, 1, 84–90. [Google Scholar] [CrossRef]
- Hodak, J.H.; Henglein, A.; Hartland, G.V. Size dependent properties of au particles: Coherent excitation and dephasing of acoustic vibrational modes. J. Chem. Phys. 1999, 111, 8613–8621. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Nikoobakht, B.; El-Sayed, M.A. How long does it take to melt a gold nanorod? A femtosecond pump-probe absorption spectroscopic study. Chem. Phys. Lett. 1999, 315, 12–18. [Google Scholar] [CrossRef]
- Kurita, H.; Takami, A.; Koda, S. Size reduction of gold particles in aqueous solution by pulsed laser irradiation. Appl. Phys. Lett. 1998, 72, 789–791. [Google Scholar] [CrossRef]
- Takami, A.; Kurita, H.; Koda, S. Laser-induced size reduction of noble metal particles. J. Phys. Chem. B 1999, 103, 1226–1232. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond dynamics of silver nanoclusters. Photoejection of electrons and fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Chang, S.S.; Shih, C.W.; Chen, C.D.; Lai, W.C.; Wang, C.R.C. The shape transition of gold nanorods. Langmuir 1999, 15, 701–709. [Google Scholar] [CrossRef]
- Chen, Y.S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.; Shefi, O. Nanometric agents in the service of neuroscience: Manipulation of neuronal growth and activity using nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Lee, N.; Kim, B.H.; Rhee, W.J.; Yoon, S.; Hyeon, T.; Park, T.H. Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials 2011, 32, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, K.; Moon, H.T.; Woo, D.G.; Yang, H.N.; Park, K.H. Electrical pulsed stimulation of surfaces homogeneously coated with gold nanoparticles to induce neurite outgrowth of PC12 cells. Langmuir 2009, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Poljak-Blazi, M.; Jaganjac, M.; Zarkovic, N. Cell Oxidative Stress: Risk of Metal Nanoparticles. In Handbook of Nanophysics: Nanomedicine and Nanorobotics; Sattler, K., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–17. [Google Scholar]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and gold nanoparticles exposure to in vitro cultured retina—Studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Haycock, J.W.; Stoddart, P.R.; McArthur, S.L. Effects of Laser-Exposed Gold Nanorods on Biochemical Pathways of Neuronal Cells. In Proceedings of the SPIE 8923: Micro/Nano Materials, Devices, and Systems, Victoria, Australia, 9 December 2013; pp. 89231A1–89231A9. [Google Scholar]
- Siddiqi, N.J.; Abdelhalim, M.A.K.; El-Ansary, A.K.; Alhomida, A.S.; Ong, W.Y. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflamm. 2012, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Thompson, A.C.; Yong, J.; Brown, W.G.A.; Stoddart, P.R. Nanoparticle-enhanced infrared neural stimulation. J. Neural Eng. 2014, 11, 065002. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.-P.; Matic, A.I.; Wells, J.D.; Jansen, E.D.; Walsh, J.T. Neural stimulation with optical radiation. Laser Photonics Rev. 2011, 5, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Stoddart, P.R.; Jansen, E.D. Optical stimulation of neurons. Curr. Mol. Imaging 2014, 3, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Farah, N.; Golan, L. Method and System for Optical Stimulation of Neurons. U.S. Patent 20,100,262,212 A1, 14 October 2010. [Google Scholar]
- Farah, N.; Zoubi, A.; Matar, S.; Golan, L.; Marom, A.; Butson, C.R.; Brosh, I.; Shoham, S. Holographically patterned activation using photo-absorber induced neural-thermal stimulation. J. Neural Eng. 2013, 10, 0506004. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Kao, C.; Mariappan, K.; Albea, J.; Jansen, E.D.; Konrad, P.; Mahadevan-Jansen, A. Optical stimulation of neural tissue in vivo. Opt. Lett. 2005, 30, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Kao, C.; Konrad, P.; Milner, T.; Kim, J.; Mahadevan-Jansen, A.; Jansen, E.D. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J. 2007, 93, 2567–2580. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Wade, S.A.; Brown, W.G.A.; Stoddart, P.R. Modeling of light absorption in tissue during infrared neural stimulation. J. Biomed. Opt. 2012, 17, 075002. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Wade, S.A.; Pawsey, N.C.; Stoddart, P.R. Infrared neural stimulation: Influence of stimulation site spacing and repetition rates on heating. IEEE Trans. Biomed. Eng. 2013, 60, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Katz, B. The effect of temperature on the electrical activity of the giant axon of the squid. J. Physiol. Lond. 1949, 109, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Cayce, J.M.; Friedman, R.M.; Jansen, E.D.; Mahavaden-Jansen, A.; Roe, A.W. Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. Neuroimage 2011, 57, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Frerck, M.J.; Holman, H.A.; Jorgensen, E.M.; Rabbitt, R.D. Exciting cell membranes with a blustering heat shock. Biophys. J. 2014, 106, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.J.; Tyler, D.J. Activation Using Infrared Light in a Mammalian Axon Model. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August 2012; pp. 1896–1899. [Google Scholar]
- Albert, E.S.; Bec, J.M.; Desmadryl, G.; Chekroud, K.; Travo, C.; Gaboyard, S.; Bardin, F.; Marc, I.; Dumas, M.; Lenaers, G.; et al. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J. Neurophys. 2012, 107, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liu, J.; Liang, S.S.; Guan, K.W.; An, L.J.; Wu, X.F.; Li, S.; Sun, C.S. Temporal modulation of sodium current kinetics in neuron cells by near-infrared laser. Cell Biochem. Biophys. 2013, 67, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Dittami, G.M.; Rajguru, S.M.; Lasher, R.A.; Hitchcock, R.W.; Rabbitt, R.D. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J. Physiol. 2011, 589, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Beier, H.T.; Tolstykh, G.P.; Musick, J.D.; Thomas, R.J.; Ibey, B.L. Plasma membrane nanoporation as a possible mechanism behind infrared excitation of cells. J. Neural Eng. 2014, 11, 066006. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, J.; Humbert, L.; Boulais, E.; Lachaine, R.; Lebrun, J.J.; Meunier, M. Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells. Biomaterials 2012, 33, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhao, Y.; Huff, T.B.; Hansen, M.N.; Wei, A.; Cheng, J.X. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv. Mater. 2007, 19, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.S.; Fedoruk, M.; Horton, M.R.; Radler, J.; Stefani, F.D.; Feldmann, J. Controlled nanometric phase transitions of phospholipid membranes by plasmonic heating of single gold nanoparticles. Nano Lett. 2009, 9, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Kirchner, S.R.; Muhlbauer, C.; Lohmuller, T.; Feldmann, J. Reversible control of current across lipid membranes by local heating. Sci. Rep. 2016, 6, 22686. [Google Scholar] [CrossRef] [PubMed]
- Barandeh, F.; Nguyen, P.L.; Kumar, R.; Iacobucci, G.J.; Kuznicki, M.L.; Kosterman, A.; Bergey, E.J.; Prasad, P.N.; Gunawardena, S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS ONE 2012, 7, e29424. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Feyen, P.; Antognazza, M.R.; Lanzani, G.; Benfenati, F. Nanoparticles: A challenging vehicle for neural stimulation. Front. Neurosci. 2016, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Stoddart, P.R. Metallic nanoparticles for peripheral nerve regeneration: Is it a feasible approach? Neural Regen. Res. 2015, 10, 1065–1066. [Google Scholar] [PubMed]
- Silva, G.A. Neuroscience nanotechnology: Progress, opportunities and challenges. Nat. Rev. Neurosci. 2006, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Bang, M.; Kim, B.S.; Lee, S.; Kotov, N.A.; Kim, B.; Jeon, D. Intracellular gold nanoparticles increase neuronal excitability and aggravate seizure activity in the mouse brain. PLoS ONE 2014, 9, e91360. [Google Scholar] [CrossRef] [PubMed]
- Boulais, E.; Lachaine, R.; Hatef, A.; Meunier, M. Plasmonics for pulsed-laser cell nanosurgery: Fundamentals and applications. J. Photochem. Photobiol. C 2013, 17, 26–49. [Google Scholar] [CrossRef]
- Li, W.W.; Chen, X.Y. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]



| Shape | Size | Plasmon Peak | Functionalization | Applications | Observed Effects |
|---|---|---|---|---|---|
| Nanorods | 48.6 nm × 13.8 nm | 780 nm | Poly(4-styrenesulfonic acid), silica | Peripheral nerve regeneration | Increased neurite length [6] |
| Nanospheres | 40 nm | - | Polyethylene glycol (PEG) | Peripheral nerve regeneration | Hind limb motor recovery, attenuation of microglial response, enhanced motor neuron protection, increased remyelination [7] |
| Nanospheres | 8.6 nm | - | Manganese-doped | Peripheral nerve regeneration | Increased neurite length [26] |
| Nanospheres | 10 nm | - | - | Integration into nerve conduits | Increased neurite length [27] |
| Nanospheres | 2–22 nm | - | - | Integration into nerve conduits | Promote adhesion and proliferation of Schwann cells [28] |
| Nanospheres | 5 nm | - | Chitosan | Integration into nerve conduits | Regeneration of the sciatic nerve [29] |
| Nanorods | Aspect ratio 3.4 | 780 nm | Silica | Modulation of electrical activity | Action potentials in primary auditory neurons [10] |
| Nanorods | 80.4 nm × 15.3 nm | 977 nm | - | Modulation of electrical activity | Action potentials in rat sciatic nerves in vivo [11] |
| Nanorods | 71.3 nm × 18.5 nm | 785 nm | Amine-terminated PEG | Modulation of electrical activity | Inhibition of neural activity in primary hippocampal neurons [12] |
| Nanospheres | 20 nm | 532 nm | Functional groups that target voltage-gated sodium, TRPV1 and P2X3 ion channels | Modulation of electrical activity | Action potentials in dorsal root ganglion cells [15] |
| Nanorods | 48.6 nm × 13.8 nm | 780 nm | Poly(4-styrenesulfonic acid) | Modulation of Ca2+ dynamics | Intracellular Ca2+ transients [8] |
| Nanorods | 60.0 nm × 15.0 nm | 780 nm | Cationic protein/lipid complex | Modulation of Ca2+ dynamics | Ca2+ influx by TRPV1 activation [29] |
| Nanorods | 82.9 nm × 13.4 nm | 982 nm | Streptavidin | Modulation of Ca2+ dynamics | Ca2+ transients in astrocytes [30] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paviolo, C.; Stoddart, P.R. Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials 2017, 7, 92. https://doi.org/10.3390/nano7040092
Paviolo C, Stoddart PR. Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials. 2017; 7(4):92. https://doi.org/10.3390/nano7040092
Chicago/Turabian StylePaviolo, Chiara, and Paul R. Stoddart. 2017. "Gold Nanoparticles for Modulating Neuronal Behavior" Nanomaterials 7, no. 4: 92. https://doi.org/10.3390/nano7040092
APA StylePaviolo, C., & Stoddart, P. R. (2017). Gold Nanoparticles for Modulating Neuronal Behavior. Nanomaterials, 7(4), 92. https://doi.org/10.3390/nano7040092