One-Dimensional Electron Transport Layers for Perovskite Solar Cells
Abstract
:1. Introduction
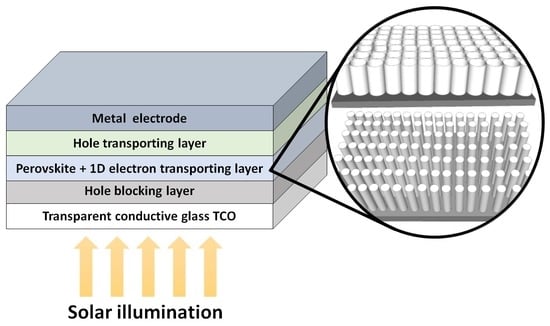
2. Architecture and Working Mechanism of Devices
3. Halide Perovskite Deposition Techniques
4. One Dimensional Nanostructures
5. Materials for One Dimensional Electron Transport Layers
6. Classes of One Dimensional Nanostructures Used as ETLs in Perovskite Solar Cells
6.1. TiO2 Nanotube Arrays
6.2. TiO2 Nanorod Arrays
6.3. ZnO Nanorod Arrays
6.4. Non-Oxide 1D-Nanostructures
7. Exploitation of Nanophotonic Effects in 1D-ETLs
8. Doping and Surface Modification
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (version 46). Prog. Photovolt. 2015, 23, 805–812. [Google Scholar] [CrossRef]
- Bhandari, K.P.; Collier, J.M.; Ellingson, R.J.; Apul, D.S. Energy payback time (EPBT) and energy return on energy invested (EROI) of solar photovoltaic systems: A systematic review and meta-analysis. Renew. Sustain. Energy Rev. 2015, 47, 133–141. [Google Scholar] [CrossRef]
- Yue, D.; You, F.; Darling, S.B. Domestic and overseas manufacturing scenarios of silicon-based photovoltaics: Life cycle energy and environmental comparative analysis. Sol. Energy 2014, 105, 669–678. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Hardin, B.E.; Snaith, H.J.; McGehee, M.D. The renaissance of dye-sensitized solar cells. Nat. Photon. 2012, 6, 162–169. [Google Scholar] [CrossRef]
- Gao, P.; Gratzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Kisslinger, R.; Hua, W.; Shankar, K. Bulk heterojunction solar cells based on blends of conjugated polymers with ii–vi and iv–vi inorganic semiconductor quantum dots. Polymers 2017, 9, 35. [Google Scholar] [CrossRef]
- Espinosa, N.; Hosel, M.; Angmo, D.; Krebs, F.C. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 2012, 5, 5117–5132. [Google Scholar] [CrossRef]
- Gong, J.; Darling, S.B.; You, F. Perovskite photovoltaics: Life-cycle assessment of energy and environmental impacts. Energy Environ. Sci. 2015, 8, 1953–1968. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Gratzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Shi, T.; Yan, Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv. Mater. 2014, 26, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A. The versatility of mesoscopic solar cells. In International Photonics and OptoElectronics, Processings of the Photonics for Energy 2015, Wuhan, China, 16–19 June 2015; OSA Publishing: Washington, DC, USA; p. PT1D.1.
- Van de Lagemaat, J.; Frank, A.J. Nonthermalized electron transport in dye-sensitized nanocrystalline TiO2 films: Transient photocurrent and random-walk modeling studies. J. Phys. Chem. B 2001, 105, 11194–11205. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.J.; Zhu, K.; Frank, A.J.; Grimes, C.A.; Mallouk, T.E. Rapid charge transport in dye-sensitized solar cells made from vertically aligned single-crystal rutile TiO2 nanowires. Angew. Chem. Int. Ed. 2012, 51, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- He, D.Q.; Sheng, X.; Yang, J.; Chen, L.P.; Zhu, K.; Feng, X.J. [1010] oriented multichannel ZnO nanowire arrays with enhanced optoelectronic device performance. J. Am. Chem. Soc. 2014, 136, 16772–16775. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; He, D.Q.; Yang, J.; Zhu, K.; Feng, X.J. Oriented assembled TiO2 hierarchical nanowire arrays with fast electron transport properties. Nano Lett. 2014, 14, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photon. 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Subbiah, A.S.; Halder, A.; Ghosh, S.; Mahuli, N.; Hodes, G.; Sarkar, S.K. Inorganic hole conducting layers for perovskite-based solar cells. J. Phys. Chem. Lett. 2014, 5, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T.-B.; Chen, C.-C.; Lu, S.; Liu, Y.; Zhou, H.; et al. Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility. ACS Nano 2014, 8, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-performance hole-extraction layer of sol–gel-processed nio nanocrystals for inverted planar perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Hou, Y.; Chen, W.; Baran, D.; Stubhan, T.; Luechinger, N.A.; Hartmeier, B.; Richter, M.; Min, J.; Chen, S.; Quiroz, C.O.R.; et al. Overcoming the interface losses in planar heterojunction perovskite-based solar cells. Adv. Mater. 2016, 28, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Ding, L. Solution-processed Cu2O and CuO as hole transport materials for efficient perovskite solar cells. Small 2015, 11, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photon. 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Bhande, S.S.; Ambade, R.B.; Shinde, D.V.; Ambade, S.B.; Patil, S.A.; Naushad, M.; Mane, R.S.; Alothman, Z.A.; Lee, S.-H.; Han, S.-H. Improved photoelectrochemical cell performance of tin oxide with functionalized multiwalled carbon nanotubes–cadmium selenide sensitizer. ACS Appl. Mater. Interfaces 2015, 7, 25094–25104. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the long-term stability of perovskite solar cells with a porous Al2O3 buffer layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Fang, G.; Wan, J.; Tao, H.; Liu, Q.; Xiong, L.; Qin, P.; Wang, J.; Lei, H.; Yang, G.; et al. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 2015, 6, 6700. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Lin, Z.-K.; Huang, W.-J.; Yang, S.-H. Wo3 nanoparticles or nanorods incorporating Cs2Co3/PCBM buffer bilayer as carriers transporting materials for perovskite solar cells. Nanoscale Res. Lett. 2016, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Yong, K. Novel cds hole-blocking layer for photostable perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 4226–4232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Yeom, E.J.; Shin, S.S.; Yang, W.S.; Lee, S.J.; Yin, W.; Kim, D.; Noh, J.H.; Ahn, T.K.; Seok, S.I. Controllable synthesis of single crystalline sn-based oxides and their application in perovskite solar cells. J. Mater. Chem. A 2017, 5, 79–86. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Kirmani, A.R.; Amassian, A. Highly efficient perovskite solar cells based on a nanostructured Wo3-TiO2 core-shell electron transporting material. J. Mater. Chem. A 2015, 3, 9051–9057. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Kelly, T.L. Compact layer free perovskite solar cells with 13.5% efficiency. J. Am. Chem. Soc. 2014, 136, 17116–17122. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Jang, I.-H.; Pellet, N.; Grätzel, M.; Park, N.-G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nano 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Wang, P.; Liu, Z.H.; Wei, L.Y.; Yang, Z.; Chen, H.R.; Fang, X.Q.; Liu, X.L.; Mai, Y.H. Controlled reaction for improved CH3NH3PbI3 transition in perovskite solar cells. Dalton Trans. 2015, 44, 17841–17849. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.N.; Mitzi, D.B.; Prikas, M.T. Synthesis and characterization of organic-inorganic perovskite thin films prepared using a versatile two-step dipping technique. Chem. Mater. 1998, 10, 403–411. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Kang, H.W.; Hsiao, S.Y.; Yang, P.F.; Chiang, K.M.; Lin, H.W. Efficient and uniform planar-type perovskite solar cells by simple sequential vacuum deposition. Adv. Mater. 2014, 26, 6647–6652. [Google Scholar] [CrossRef] [PubMed]
- Bhachu, D.S.; Scanlon, D.O.; Saban, E.J.; Bronstein, H.; Parkin, I.P.; Carmalt, C.J.; Palgrave, R.G. Scalable route to CH3NH3PbI3 perovskite thin films by aerosol assisted chemical vapour deposition. J. Mater. Chem. A 2015, 3, 9071–9073. [Google Scholar] [CrossRef]
- Wei, Z.H.; Chen, H.N.; Yan, K.Y.; Yang, S.H. Inkjet printing and instant chemical transformation of a CH3NH3PbI3/nanocarbon electrode and interface for planar perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 13239–13243. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Jiang, K.J.; Su, M.J.; Cui, X.P.; Huang, J.H.; Zhang, Q.Q.; Zhou, X.Q.; Yang, L.M.; Song, Y.L. Inkjet printing of CH3NH3PbI3 on a mesoscopic TiO2 film for highly efficient perovskite solar cells. J. Mater. Chem. A 2015, 3, 9092–9097. [Google Scholar] [CrossRef]
- Barrows, A.T.; Pearson, A.J.; Kwak, C.K.; Dunbar, A.D.F.; Buckley, A.R.; Lidzey, D.G. Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition. Energy Environ. Sci. 2014, 7, 2944–2950. [Google Scholar] [CrossRef]
- Boopathi, K.M.; Ramesh, M.; Perumal, P.; Huang, Y.C.; Tsao, C.S.; Chen, Y.F.; Lee, C.H.; Chu, C.W. Preparation of metal halide perovskite solar cells through a liquid droplet assisted method. J. Mater. Chem. A 2015, 3, 9257–9263. [Google Scholar] [CrossRef]
- Hwang, K.; Jung, Y.S.; Heo, Y.J.; Scholes, F.H.; Watkins, S.E.; Subbiah, J.; Jones, D.J.; Kim, D.Y.; Vak, D. Toward large scale roll-to-roll production of fully printed perovskite solar cells. Adv. Mater. 2015, 27, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Dainy, G.A.; Bourdo, S.E.; Saini, V.; Berry, B.C.; Biris, A.S. Hybrid perovskite photovoltaic devices: Properties, architecture and fabrication methods. Energy Technol. 2017, 5, 373–401. [Google Scholar] [CrossRef]
- Bi, D.; Boschloo, G.; Schwarzmuller, S.; Yang, L.; Johansson, E.M.J.; Hagfeldt, A. Efficient and stable CH3NH3PbI3-sensitized zno nanorod array solid-state solar cells. Nanoscale 2013, 5, 11686–11691. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Cai, B.; Wang, X.; Yang, Z.; Xing, Y.; Miao, S.; Zhang, W.-H.; Li, C. Synthesis of oriented TiO2 nanocones with fast charge transfer for perovskite solar cells. Nano Energy 2015, 11, 409–418. [Google Scholar] [CrossRef]
- Gao, X.F.; Li, J.Y.; Baker, J.; Hou, Y.; Guan, D.S.; Chen, J.H.; Yuan, C. Enhanced photovoltaic performance of perovskite CH3NH3PbI3 solar cells with freestanding TiO2 nanotube array films. Chem. Commun. 2014, 50, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wiranwetchayan, O.; Zhang, Q.; Liang, Z.; Sun, Y.; Cao, G. Growth of single-crystalline rutile TiO2 nanorods on fluorine-doped tin oxide glass for organic–inorganic hybrid solar cells. J. Mater. Sci. Mater. Electron. 2012, 23, 1657–1663. [Google Scholar] [CrossRef]
- Thakur, U.K.; Askar, A.M.; Kisslinger, R.; Wiltshire, B.D.; Kar, P.; Shankar, K. Halide perovskite solar cells using monocrystalline TiO2 nanorod arrays as electron transport layers: Impact of nanorod morphology. Nanotechnology 2017. submitted for publication. [Google Scholar]
- Mahmood, K.; Swain, B.S.; Amassian, A. 16.1% efficient hysteresis-free mesostructured perovskite solar cells based on synergistically improved ZnO nanorod arrays. Adv. Energy Mater. 2015, 5, 1500568. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. Core-shell heterostructured metal oxide arrays enable superior light-harvesting and hysteresis-free mesoscopic perovskite solar cells. Nanoscale 2015, 7, 12812–12819. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Swain, B.S.; Han, G.-S.; Kim, B.-J.; Jung, H.S. Polyethylenimine-assisted growth of high-aspect-ratio nitrogen-doped ZnO (nzo) nanorod arrays and their effect on performance of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 10028–10043. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Worfolk, B.J.; Rider, D.A.; Taschuk, M.T.; Buriak, J.M.; Brett, M.J. C60 fullerene nanocolumns–polythiophene heterojunctions for inverted organic photovoltaic cells. ACS Appl. Mater. Interfaces 2011, 3, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Li, W.; Bo, Z.S.; Brett, M.J. Inverted photovoltaic cells of nanocolumnar C60 filled with solution processed small molecule 3–Q. Org. Electron. 2012, 13, 2647–2652. [Google Scholar] [CrossRef]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2011, 23, 682–732. [Google Scholar] [CrossRef]
- Bera, A.; Sheikh, A.D.; Haque, M.A.; Bose, R.; Alarousu, E.; Mohammed, O.F.; Wu, T. Fast crystallization and improved stability of perovskite solar cells with Zn2SnO4 electron transporting layer: Interface matters. ACS Appl. Mater. Interfaces 2015, 7, 28404–28411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Z.; Shao, Z.P.; Ye, J.J.; Zhang, X.H.; Pan, X.; Dai, S.Y. Mesoporous BaSnO3 layer based perovskite solar cells. Chem. Commun. 2016, 52, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Wu, K.W.; Sheikh, A.; Alarousu, E.; Mohammed, O.F.; Wu, T. Perovskite oxide SrTiO3 as an efficient electron transporter for hybrid perovskite solar cells. J. Phys. Chem. C 2014, 118, 28494–28501. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; Coss, R.d.; Oskam, G. Phase-pure tio 2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G.; van de Lagemaat, J.; Frank, A.J. Comparison of dye-sensitized rutile- and anatase-based TiO2 solar cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinès, R.; Schmid, P.E.; Lévy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Yella, A.; Heiniger, L.P.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Nanocrystalline rutile electron extraction layer enables low-temperature solution processed perovskite photovoltaics with 13.7% efficiency. Nano Lett. 2014, 14, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Kar, P.; Wiltshire, B.D.; Askar, A.M.; Shankar, K. Electron transport, trapping and recombination in anodic TiO2 nanotube arrays. Curr. Nanosci. 2015, 11, 593–614. [Google Scholar] [CrossRef]
- Benlamri, M.; Farsinezhad, S.; Barlage, D.W.; Shankar, K. Low residual donor concentration and enhanced charge transport in low-cost electrodeposited ZnO. J. Mater. Chem. C 2016, 4, 2279–2283. [Google Scholar] [CrossRef]
- Shoichiro, N.; Naoomi, Y.; Taro, H.; Yasushi, H.; Toshihiro, S.; Tetsuya, H. High mobility exceeding 80 cm2 v−1 s−1 in polycrystalline ta-doped SnO2 thin films on glass using anatase TiO2 seed layers. Appl. Phys. Express 2010, 3, 031102. [Google Scholar]
- Wang, P.; Zhao, J.; Liu, J.; Wei, L.; Liu, Z.; Guan, L.; Cao, G. Stabilization of organometal halide perovskite films by SnO2 coating with inactive surface hydroxyl groups on ZnO nanorods. J. Power Sources 2017, 339, 51–60. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.L.; Ke, W.J.; Fang, G.J. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Application of highly-ordered TiO2 nanotube-arrays in heterojunction dye-sensitized solar cells. J. Phys. D Appl. Phys. 2006, 39, 2498–2503. [Google Scholar] [CrossRef]
- Bandara, J.; Shankar, K.; Basham, J.; Wietasch, H.; Paulose, M.; Varghese, O.K.; Grimes, C.A.; Thelakkat, M. Integration of TiO2 nanotube arrays into solid-state dye-sensitized solar cells. Eur. Phys. J. Appl. Phys. 2011, 53, 20601. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe−TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. High efficiency double heterojunction polymer photovoltaic cells using highly ordered TiO2 nanotube arrays. Appl. Phys. Lett. 2007, 91, 152111. [Google Scholar] [CrossRef]
- Kim, S.; Mor, G.K.; Paulose, M.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Broad spectrum light harvesting in TiO2 nanotube array—Hemicyanine dye-P3HT hybrid solid-state solar cells. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 1573–1580. [Google Scholar] [CrossRef]
- Jijun, Q.; Weidong, Y.; Xiangdong, G.; Xiaomin, L. Sol–gel assisted ZnO nanorod array template to synthesize TiO2 nanotube arrays. Nanotechnology 2006, 17, 4695. [Google Scholar]
- Sander, M.S.; Côté, M.J.; Gu, W.; Kile, B.M.; Tripp, C.P. Template-assisted fabrication of dense, aligned arrays of titania nanotubes with well-controlled dimensions on substrates. Adv. Mater. 2004, 16, 2052–2057. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Zhang, X.Y.; Zhang, W.F.; Yang, Z.Y.; Wang, N. Formation mechanism of TiO2 nanotubes. Appl. Phys. Lett. 2003, 82, 281–283. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Transparent highly ordered TiO2 nanotube arrays via anodization of titanium thin films. Adv. Funct. Mater. 2005, 15, 1291–1296. [Google Scholar] [CrossRef]
- Galstyan, V.; Vomiero, A.; Concina, I.; Braga, A.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Vertically aligned TiO2 nanotubes on plastic substrates for flexible solar cells. Small 2011, 7, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Farsinezhad, S.; Mohammadpour, A.; Dalrymple, A.N.; Geisinger, J.; Kar, P.; Brett, M.J.; Shankar, K. Transparent anodic TiO2 nanotube arrays on plastic substrates for disposable biosensors and flexible electronics. J. Nanosci. Nanotechnol. 2013, 13, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Grimes, C.A. Formation of vertically oriented TiO2 nanotube arrays using a fluoride free HCl aqueous electrolyte. J. Phys. Chem. C 2007, 111, 13028–13032. [Google Scholar] [CrossRef]
- Nakayama, K.; Kubo, T.; Nishikitani, Y. Anodic formation of titania nanotubes with ultrahigh aspect ratio. Electrochem. Solid–State Lett. 2008, 11, C23–C26. [Google Scholar] [CrossRef]
- Berger, S.; Kunze, J.; Schmuki, P.; LeClere, D.; Valota, A.T.; Skeldon, P.; Thompson, G.E. A lithographic approach to determine volume expansion factors during anodization: Using the example of initiation and growth of TiO2-nanotubes. Electrochim. Acta 2009, 54, 5942–5948. [Google Scholar] [CrossRef]
- Chen, B.; Hou, J.; Lu, K. Formation mechanism of TiO2 nanotubes and their applications in photoelectrochemical water splitting and supercapacitors. Langmuir 2013, 29, 5911–5919. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Russo, V.; Li Bassi, A.; Di Fonzo, F.; Murray, T.M.; Efstathiadis, H.; Agnoli, A.; Kunze-Liebhäuser, J. TiO2 nanotubes: Interdependence of substrate grain orientation and growth rate. ACS Appl. Mater. Interfaces 2015, 7, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Chen, X.; Schriver, M.; Suen, T.; Mao, S.S. Fabrication of 10 nm diameter TiO2 nanotube arrays by titanium anodization. Thin Solid Films 2007, 515, 8511–8514. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A new benchmark for TiO2 nanotube array growth by anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Shankar, K. Anodic TiO2 nanotube arrays with optical wavelength-sized apertures. J. Mater. Chem. 2010, 20, 8474–8477. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2003, 18, 156–165. [Google Scholar] [CrossRef]
- Lee, S.; Park, I.J.; Kim, D.H.; Seong, W.M.; Kim, D.W.; Han, G.S.; Kim, J.Y.; Jung, H.S.; Hong, K.S. Crystallographically preferred oriented TiO2 nanotube arrays for efficient photovoltaic energy conversion. Energy Environ. Sci. 2012, 5, 7989–7995. [Google Scholar] [CrossRef]
- John K, A.; Naduvath, J.; Mallick, S.; Shripathi, T.; Thankamoniamma, M.; Philip, R.R. A novel cost effective fabrication technique for highly preferential oriented TiO2 nanotubes. Nanoscale 2015, 7, 20386–20390. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, G.; Fong, H.; Zhu, Z. Electron transport and recombination in photoanode of electrospun TiO2 nanotubes for dye-sensitized solar cells. J. Phys. Chem. C 2013, 117, 1641–1646. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Paulose, M.; Dar, M.I.; Moehl, T.; Arora, N.; Gao, P.; Varghese, O.K.; Gatzel, M.; Nazeeruddin, M.K. Stable and efficient perovskite solar cells based on titania nanotube arrays. Small 2015, 11, 5533–5539. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Gollon, S.; Qiu, M.; Guan, D.; Guo, X.; Chen, J.; Yuan, C. A TiO2 nanotube network electron transport layer for high efficiency perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 4956–4961. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Song, S.; Hörantner, M.T.; Snaith, H.J.; Park, T. Well-defined nanostructured, single-crystalline TiO2 electron transport layer for efficient planar perovskite solar cells. ACS Nano 2016, 10, 6029–6036. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, Z.; Xu, W.J.; Kulkarni, S.A.; Batabyal, S.K.; Zhang, S.; Cao, A.Y.; Wong, L.H. TiO2 nanotube arrays based flexible perovskite solar cells with transparent carbon nanotube electrode. Nano Energy 2015, 11, 728–735. [Google Scholar] [CrossRef]
- Salazar, R.; Altomare, M.; Lee, K.; Tripathy, J.; Kirchgeorg, R.; Nguyen, N.T.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Schmuki, P. Use of anodic TiO2 nanotube layers as mesoporous scaffolds for fabricating CH3NH3PbI3 perovskite-based solid-state solar cells. Chemelectrochem 2015, 2, 824–828. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, Y.; Yan, K.; Zhong, M.; Mu, C.; Yan, H.; Yang, S. All-solid-state hybrid solar cells based on a new organometal halide perovskite sensitizer and one-dimensional TiO2 nanowire arrays. Nanoscale 2013, 5, 3245–3248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.-G. High efficiency solid-state sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Sheng, X.; Li, Y.; Feng, X.; Xu, T. Rutile TiO2 nanowire-based perovskite solar cells. Chem. Commun. 2014, 50, 14720–14723. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guo, R.; Kadel, K.; Liu, Y.; O'Shea, K.; Bone, R.; Wang, X.; He, J.; Li, W. Improved charge transport of Nb-doped TiO2 nanorods in methylammonium lead iodide bromide perovskite solar cells. J. Mater. Chem. A 2014, 2, 19616–19622. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Z.; Tao, X.; Sun, H.; Chen, W.; Zhou, X. Sn-doped TiO2 nanorod arrays and application in perovskite solar cells. RSC Adv. 2014, 4, 64001–64005. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin atomic layer deposited TiO2 for surface passivation of hydrothermally grown 1D TiO2 nanorod arrays for efficient solid-state perovskite solar cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Li, X.; Dai, S.-M.; Zhu, P.; Deng, L.-L.; Xie, S.-Y.; Cui, Q.; Chen, H.; Wang, N.; Lin, H. Efficient perovskite solar cells depending on TiO2 nanorod arrays. ACS Appl. Mater. Interfaces 2016, 8, 21358–21365. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kwon, S.G.; Yu, T.; Cho, M.; Lee, J.; Yoon, J.; Hyeon, T. Large-scale synthesis of TiO2 nanorods via nonhydrolytic sol–gel ester elimination reaction and their application to photocatalytic inactivation of E. coli. J. Phys. Chem. B 2005, 109, 15297–15302. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Reucroft, P.J.; Yang, F.; Dozier, A. Growth of TiO2 nanorods by metalorganic chemical vapor deposition. J. Cryst. Growth 2003, 256, 83–88. [Google Scholar] [CrossRef]
- Wu, J.-J.; Yu, C.-C. Aligned TiO2 nanorods and nanowalls. J. Phys. Chem. B 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Chen, C.A.; Chen, Y.M.; Korotcov, A.; Huang, Y.S.; Tsai, D.S.; Tiong, K.K. Growth and characterization of well-aligned densely-packed rutile TiO2 nanocrystals on sapphire substrates via metal–organic chemical vapor deposition. Nanotechnology 2008, 19, 075611. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Park, K.S.; Kim, T.G.; Choi, H.J.; Sung, Y.M. Controlled growth of high-quality TiO2 nanowires on sapphire and silica. Nanotechnology 2006, 17, 4317. [Google Scholar] [CrossRef]
- Zhuge, F.; Yanagida, T.; Nagashima, K.; Yoshida, H.; Kanai, M.; Xu, B.; Klamchuen, A.; Meng, G.; He, Y.; Rahong, S.; et al. Fundamental strategy for creating vls grown TiO2 single crystalline nanowires. J. Phys. Chem. C 2012, 116, 24367–24372. [Google Scholar] [CrossRef]
- Qing, H.; Lian, G. A simple route for the synthesis of rutile TiO2 nanorods. Chem. Lett. 2003, 32, 638–639. [Google Scholar]
- Byrappa, K.; Yoshimura, M. Hydrothermal technology—Principles and applications. In Handbook of Hydrothermal Technology, 2nd ed.; William Andrew Publishing: Oxford, UK, 2013; pp. 1–49. [Google Scholar]
- Feng, X.J.; Shankar, K.; Varghese, O.K.; Paulose, M.; Latempa, T.J.; Grimes, C.A. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis details and applications. Nano Lett. 2008, 8, 3781–3786. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Chen, D.; Huang, F.; Cheng, Y.-B.; Caruso, R.A. Sub-100°C solution processed amorphous titania nanowire thin films for high-performance perovskite solar cells. J. Power Sources 2016, 329, 17–22. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Di Giacomo, F.; Palma, A.L.; Matteocci, F.; Ahmed, I.; Razza, S.; D’Epifanio, A.; Licoccia, S.; Ismail, J.; Di Carlo, A.; et al. Vertical TiO2 nanorods as a medium for stable and high-efficiency perovskite solar modules. ACS Nano 2015, 9, 8420–8429. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye-sensitized solar cells. ACS Nano 2014, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.H.; Yantara, N.; Dharani, S.; Graetzel, M.; Mhaisalkar, S.; Boix, P.P.; Mathews, N. Flexible, low-temperature, solution processed ZnO-based perovskite solid state solar cells. Chem. Commun. 2013, 49, 11089–11091. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, Y.; Shi, J.; Wei, H.; Xiao, J.; Xu, X.; Luo, J.; Xu, J.; Li, D.; Luo, Y.; et al. Impressive enhancement in the cell performance of ZnO nanorod-based perovskite solar cells with Al-doped ZnO interfacial modification. Chem. Commun. 2014, 50, 13381–13384. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Z.; Cai, L.; Chen, W.; Wang, B.; Chen, K.; Bai, H.; Tian, Q.; Fan, B. Magnetron sputtered zinc oxide nanorods as thickness-insensitive cathode interlayer for perovskite planar-heterojunction solar cells. ACS Appl. Mater. Interfaces 2014, 6, 20585–20589. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Swain, B.S.; Amassian, A. Double-layered zno nanostructures for efficient perovskite solar cells. Nanoscale 2014, 6, 14674–14678. [Google Scholar] [CrossRef] [PubMed]
- Dymshits, A.; Iagher, L.; Etgar, L. Parameters influencing the growth of ZnO nanowires as efficient low temperature flexible perovskite-based solar cells. Materials 2016, 9, 60. [Google Scholar] [CrossRef]
- Wang, H.; Yan, L.; Liu, J.; Li, J.; Wang, H. Fabrication of well-aligned ZnO nanorod photoanodes for perovskite solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 6872–6880. [Google Scholar] [CrossRef]
- Wang, B.X.; Liu, T.F.; Zhou, Y.B.; Chen, X.; Yuan, X.B.; Yang, Y.Y.; Liu, W.P.; Wang, J.M.; Han, H.W.; Tang, Y.W. Hole-conductor-free perovskite solar cells with carbon counter electrodes based on ZnO nanorod arrays. Phys. Chem. Chem. Phys. 2016, 18, 27078–27082. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Yu, J.; Feng, B.; Zhou, H.; Zhang, J.; Duan, J.; Fan, X.; Aken, P.A.V.; Lund, P.D.; et al. Performance improvement of perovskite solar cells based on pcbm-modified ZnO-nanorod arrays. IEEE J. Photovolt. 2016, 6, 1530–1536. [Google Scholar] [CrossRef]
- Catano, F.A.; Allende, L.W.; Gomez, H. Electrodeposition of ZnO nanorod arrays for application in perovskite based solar cells. J. Chil. Chem. Soc. 2015, 60, 2940–2943. [Google Scholar]
- Zhang, J.; Barboux, P.; Pauporté, T. Electrochemical design of nanostructured ZnO charge carrier layers for efficient solid-state perovskite-sensitized solar cells. Adv. Energy Mater. 2014, 4, 1400932. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional zno nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Sugunan, A.; Warad, H.C.; Boman, M.; Dutta, J. Zinc oxide nanowires in chemical bath on seeded substrates: Role of hexamine. J. Sol Gel Sci. Technol. 2006, 39, 49–56. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yassitepe, E.; Voznyy, O.; Janmohamed, A.; Lan, X.; Levina, L.; Comin, R.; Sargent, E.H. Self-assembled pbse nanowire: Perovskite hybrids. J. Am. Chem. Soc. 2015, 137, 14869–14872. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, C.; Wang, J.; Zhang, X.; Ren, X. A high-efficiency Si nanowire array/perovskite hybrid solar cell. Nanoscale Res. Lett. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.E.I.; Ren, X.; Chen, L.; Choy, W.C.H. The efficiency limit of CH3NH3PbI3 perovskite solar cells. Appl. Phys. Lett. 2015, 106, 221104. [Google Scholar] [CrossRef]
- Du, Q.G.; Shen, G.; John, S. Light-trapping in perovskite solar cells. AIP Adv. 2016, 6, 065002. [Google Scholar] [CrossRef]
- Yeh, L.-K.; Lai, K.-Y.; Lin, G.-J.; Fu, P.-H.; Chang, H.-C.; Lin, C.-A.; He, J.-H. Giant efficiency enhancement of gaas solar cells with graded antireflection layers based on syringelike ZnO nanorod arrays. Adv. Energy Mater. 2011, 1, 506–510. [Google Scholar] [CrossRef]
- Strano, V.; Smecca, E.; Depauw, V.; Trompoukis, C.; Alberti, A.; Reitano, R.; Crupi, I.; Gordon, I.; Mirabella, S. Low-cost high-haze films based on zno nanorods for light scattering in thin C–Si solar cells. Appl. Phys. Lett. 2015, 106, 013901. [Google Scholar] [CrossRef]
- Nowak, R.-E.; Vehse, M.; Sergeev, O.; von Maydell, K.; Agert, C. Zno nanorod arrays as light trapping structures in amorphous silicon thin-film solar cells. Sol. Energy Mater. Sol. Cells 2014, 125, 305–309. [Google Scholar] [CrossRef]
- Shinagawa, T.; Shibata, K.; Shimomura, O.; Chigane, M.; Nomura, R.; Izaki, M. Solution-processed high-haze ZnO pyramidal textures directly grown on a tco substrate and the light-trapping effect in Cu2O solar cells. J. Mater. Chem. C 2014, 2, 2908–2917. [Google Scholar] [CrossRef]
- Lin, S.-H.; Su, Y.-H.; Cho, H.-W.; Kung, P.-Y.; Liao, W.-P.; Wu, J.-J. Nanophotonic perovskite solar cell architecture with a three-dimensional TiO2 nanodendrite scaffold for light trapping and electron collection. J. Mater. Chem. A 2016, 4, 1119–1125. [Google Scholar] [CrossRef]
- Cui, Y.; van Dam, D.; Mann, S.A.; van Hoof, N.J.J.; van Veldhoven, P.J.; Garnett, E.C.; Bakkers, E.P.A.M.; Haverkort, J.E.M. Boosting solar cell photovoltage via nanophotonic engineering. Nano Lett. 2016, 16, 6467–6471. [Google Scholar] [CrossRef] [PubMed]
- Manseki, K.; Ikeya, T.; Tamura, A.; Ban, T.; Sugiura, T.; Yoshida, T. Mg-doped TiO2 nanorods improving open-circuit voltages of ammonium lead halide perovskite solar cells. RSC Adv. 2014, 4, 9652–9655. [Google Scholar] [CrossRef]
- Archana, P.S.; Jose, R.; Jin, T.M.; Vijila, C.; Yusoff, M.M.; Ramakrishna, S. Structural and electrical properties of Nb-doped anatase TiO2 nanowires by electrospinning. J. Am. Ceram. Soc. 2010, 93, 4096–4102. [Google Scholar] [CrossRef]
- Lü, X.; Yang, W.; Quan, Z.; Lin, T.; Bai, L.; Wang, L.; Huang, F.; Zhao, Y. Enhanced electron transport in Nb-doped TiO2 nanoparticles via pressure-induced phase transitions. J. Am. Chem. Soc. 2014, 136, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Groff, A.; Sorarù, G.D.; Clemens, F.J. Suppressing deep traps in self-organized TiO2 nanotubes by nb doping and optimized water content. J. Electrochem. Soc. 2016, 163, H243–H251. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, N.; Liu, Q.; Fang, Y.; Zhou, X.; Zhang, J.; Lin, Y. Sn-doped TiO2 photoanode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 8888–8893. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Niu, L.; Liu, J.; Zhou, X. TiO2 nanorod arrays modified with SnO2–Sb2O3 nanoparticles and application in perovskite solar cell. Thin Solid Films 2017, 621, 6–11. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Madaria, A.R.; Zhou, C. Growth of aligned single-crystalline rutile TiO2 nanowires on arbitrary substrates and their application in dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 7787–7792. [Google Scholar] [CrossRef]
- Tao, H.; Fang, G.-j.; Ke, W.-j.; Zeng, W.; Wang, J. In-situ synthesis of TiO2 network nanoporous structure on Ti wire substrate and its application in fiber dye sensitized solar cells. J. Power Sources 2014, 245, 59–65. [Google Scholar] [CrossRef]
- Tao, H.; Ke, W.; Wang, J.; Liu, Q.; Wan, J.; Yang, G.; Fang, G. Perovskite solar cell based on network nanoporous layer consisted of TiO2 nanowires and its interface optimization. J. Power Sources 2015, 290, 144–152. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Yang, S.-H. Improved efficiency of perovskite solar cells based on Ni-doped ZnO nanorod arrays and li salt-doped P3HT layer for charge collection. Opt. Mater. Express 2016, 6, 3651–3669. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; Hayase, S. All-solid perovskite solar cells with HOCO-R-NH3+I– anchor-group inserted between porous titania and perovskite. J. Phys. Chem. C 2014, 118, 16651–16659. [Google Scholar] [CrossRef]
- Shih, Y.C.; Wang, L.Y.; Hsieh, H.C.; Lin, K.F. Enhancing the photocurrent of perovskite solar cells via modification of the TiO2/CH3NH3PbI3 heterojunction interface with amino acid. J. Mater. Chem. A 2015, 3, 9133–9136. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Liang, Z.; Gao, D.; Huang, W. Interfacial engineering by using self-assembled monolayer in mesoporous perovskite solar cell. RSC Adv. 2015, 5, 94290–94295. [Google Scholar] [CrossRef]
- Cao, J.; Yin, J.; Yuan, S.; Zhao, Y.; Li, J.; Zheng, N. Thiols as interfacial modifiers to enhance the performance and stability of perovskite solar cells. Nanoscale 2015, 7, 9443–9447. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Farsinezhad, S.; Wiltshire, B.D.; Shankar, K. Majority carrier transport in single crystal rutile nanowire arrays. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2014, 8, 512–516. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Mohammadpour, A.; Farsinezhad, S.; Wiltshire, B.D.; Nosrati, M.; Askar, A.M.; Daneshmand, M.; Shankar, K. Time-resolved microwave photoconductivity (TRMC) using planar microwave resonators: Application to the study of long-lived charge pairs in photoexcited titania nanotube arrays. J. Phys. Chem. C 2015, 119, 14358–14365. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Wiltshire, B.D.; Zhang, Y.; Farsinezhad, S.; Askar, A.M.; Kisslinger, R.; Ren, Y.; Kar, P.; Shankar, K. 100-fold improvement in carrier drift mobilities in alkanephosphonate-passivated monocrystalline TiO2 nanowire arrays. Nanotechnology 2017, 28, 144001. [Google Scholar] [CrossRef] [PubMed]














| Year | Device Structure | VOC (V) | JSC (mA·cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| 2013 | FTO|TiO2 BL|TiO2 NR|MAPbI3|Spiro|Au | 0.82 | 10.1 | 0.59 | 4.87 | [110] |
| 2013 | FTO|TiO2 BL|TiO2 NR|MAPbI3|Spiro|Au | 0.96 | 15.6 | 0.63 | 9.40 | [111] |
| 2014 | FTO|TiO2 BL|TiO2 NR|MAPbI3|Spiro|Au | 0.77 | 22.3 | 0.68 | 11.7 | [112] |
| 2014 | FTO|TIO2 BL|TIO2 NT| MAPbI3|Spiro | 0.63 | 17.9 | 0.58 | 6.52 | [57] |
| 2014 | FTO|TiO2 BL|Nb-TiO2 NR|MAPbIxBr3-x|Spiro|Au | 0.87 | 16.5 | 0.52 | 7.50 | [113] |
| 2014 | FTO|TiO2 BL|Sn-TiO2 NR|MAPbI3|Spiro|Ag | 0.74 | 14.9 | 0.52 | 6.31 | [114] |
| 2015 | FTO|TiO2 BL|TiO2 NR/Layer|MAPbI3|Spiro|Au | 0.95 | 19.8 | 0.72 | 13.5 | [115,116] |
| 2015 | FTO|TiO2 NT|MAPbI3|Spiro|Au | 1.07 | 22.6 | 0.64 | 14.8 | [105] |
| 2015 | FTO|TiO2 BL|TIO2 NT|MAPbI3|Au | 0.67 | 19.6 | 0.37 | 5.00 | [109] |
| 2015 | Ti|TiO2 NT|MAPbI3|CNT Film|Spiro | 0.99 | 14.4 | 0.68 | 8.31 | [108] |
| 2016 | FTO|TiO2 BL|TiO2 NR|MAPbI3|Spiro|Au | 1.04 | 22.9 | 0.76 | 18.2 | [116] |
| 2016 | FTO|TiO2 NT|MAPbI3-xAcx|Ag | 1.06 | 20.5 | 0.7 | 15.2 | [107] |
| 2017 | FTO|TiO2 BL|TiO2 NT network|MAPbI3|Ag | 0.88 | 24.8 | 0.63 | 13.8 | [106] |
| Year | Device Structure | VOC (V) | JSC (mA·cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| 2013 | FTO|ZnO NW|MAPbI3|Spiro|Ag | 0.68 | 12.7 | 0.58 | 5.00 | [55] |
| 2013 | FTO|ZnO SL|ZnO NW|MAPbI3|Spiro|Au | 1.02 | 17.0 | 0.51 | 8.90 | [130] |
| 2014 | FTO|ZnO SL|ZnO NW|AZO|MAPbI3|Spiro|Au | 0.90 | 19.8 | 0.60 | 10.7 | [131] |
| 2014 | FTO|ZnO SL|ZnO NW|MAPbI3|Spiro|Au | 0.99 | 20.1 | 0.56 | 11.1 | [132] |
| 2014 | FTO|ZnO NR|MAPbI3|Spiro|MoO3|Ag | 1.04 | 22.4 | 0.57 | 13.4 | [133] |
| 2014 | FTO|TiO2|ZnO SL|ZnO NW|MAPbI3|Spiro|Ag | 0.93 | 18.0 | 0.62 | 10.4 | [134] |
| 2014 | FTO|ZnO NR|PEI|MAPbI3|Spiro|Ag | 0.97 | 21.7 | 0.70 | 16.2 | [60] |
| 2015 | FTO|ZnO NW|TiO2 core shell|MAPbI3|Spiro|Au | 1.00 | 22.0 | 0.70 | 15.4 | [61] |
| 2016 | FTO|ZnO SL|ZnO NR|MAPbI3|Spiro|Au | 0.68 | 21.6 | 0.62 | 9.06 | [135] |
| 2016 | FTO|ZnO NR|SnO2|Spiro|Ag | 0.90 | 23.3 | 0.57 | 12.2 | [76] |
| 2016 | AZO|ZnO NR|MAPbIxCl3-x|Carbon | 0.86 | 14.9 | 0.28 | 3.62 | [136] |
| 2016 | FTO|ZnO-TiO2 NW|ZrO2|MAPbI3|Carbon | 0.96 | 14.8 | 0.58 | 8.24 | [137] |
| 2016 | ITO|Ni-ZnO NW|PCBM|(MA)x(GA)1-xPbI3|P3HT|Au | 0.83 | 23.7 | 0.70 | 13.8 | [34] |
| 2016 | FTO|ZnO SL|ZnO NW|PCBM| MAPbI3|Spiro|Au | 0.96 | 22.1 | 0.55 | 11.7 | [138] |
| Year | Device Structure | VOC (V) | JSC (mA·cm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| 2016 | FTO|ZnO-TiO2 NW|ZrO2|MAPbI3|Carbon | 0.96 | 14.8 | 0.58 | 8.24 | [137] |
| 2016 | ITO|Ni-ZnO NW|PCBM|(MA)x(GA)1-xPbI3|P3HT|Au | 0.83 | 23.7 | 0.70 | 13.8 | [34] |
| 2015 | FTO|ZnO NW|TiO2 core shell|MAPbI3|Spiro|Au | 1.00 | 22.0 | 0.70 | 15.4 | [61] |
| 2014 | FTO|TiO2|ZnO SL|ZnO NW|MAPbI3|Spiro|Ag | 0.93 | 18.0 | 0.62 | 10.4 | [134] |
| 2014 | FTO|TiO2 BL/SL|Nb-TiO2 NR|MAPbIxBr1-x|Spiro|Au | 0.87 | 16.5 | 0.52 | 7.50 | [113] |
| 2014 | FTO|TiO2 SL|Sn-TiO2 NR|MAPbI3|Spiro|Ag | 0.74 | 14.9 | 0.52 | 6.31 | [114] |
| 2015 | FTO|TiO2 BL|TiO2 NR/Layer|MAPbI3|Spiro|Au | 0.95 | 19.8 | 0.72 | 13.5 | [115] |
| 2015 | FTO|WO3 BL|WO3 NR|TiO2|MAPbI3|Spiro|Ag | 0.86 | 15.00 | 0.70 | 9.10 | [38] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, U.K.; Kisslinger, R.; Shankar, K. One-Dimensional Electron Transport Layers for Perovskite Solar Cells. Nanomaterials 2017, 7, 95. https://doi.org/10.3390/nano7050095
Thakur UK, Kisslinger R, Shankar K. One-Dimensional Electron Transport Layers for Perovskite Solar Cells. Nanomaterials. 2017; 7(5):95. https://doi.org/10.3390/nano7050095
Chicago/Turabian StyleThakur, Ujwal K., Ryan Kisslinger, and Karthik Shankar. 2017. "One-Dimensional Electron Transport Layers for Perovskite Solar Cells" Nanomaterials 7, no. 5: 95. https://doi.org/10.3390/nano7050095
APA StyleThakur, U. K., Kisslinger, R., & Shankar, K. (2017). One-Dimensional Electron Transport Layers for Perovskite Solar Cells. Nanomaterials, 7(5), 95. https://doi.org/10.3390/nano7050095