Magnetic Nanoparticles for Antibiotics Detection
Abstract
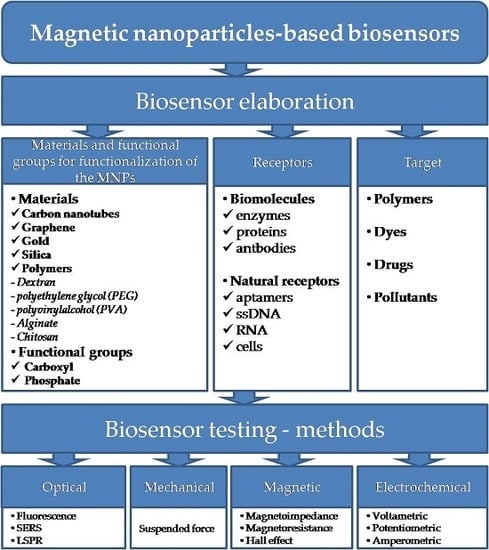
:1. Introduction
2. Synthesis and Characterization of MNPs
- Oxides (ferrites), also known as iron oxide nanoparticles, are the most common and widely used MNPs. There are eight iron oxides known, three of them being very popular: hematite (α-Fe2O3), maghemite (γ-Fe2O3) and magnetite (Fe3O4) for specific technical and biomedical applications [33]. If the diameter of the particles is maintained smaller than 128 nm, the self agglomeration of the particles can be avoided while this MNPs exhibits magnetic behavior only in the presence of an external magnetic field. This is due to the supermagnetic properties of these MNPs [34]. The surface of ferrite based nanoparticles can be often modified by different compounds, such as surfactants, silica and other derivatives in order to increase their stability in aqueous media [35]. An example of this type of MNPs is presented in Figure 1a [36].
- Metallic nanoparticles, present only the metallic core, and can be more suitable for biomedical applications due to their higher magnetic moment versus oxides. Important drawbacks include the properties of being pyrophoric, and presence of high reactivity to oxidizing agents.
- Metallic nanoparticles with a shell, consisting of the coverage of metallic core with a shell obtained after gentle oxidation or reaction with surfactants, polymers or precious metals. For example, Co nanoparticles were covered with an anti-ferromagnetic CoO layer formed in the presence of oxygen environment or, one with a shell made of graphene (Figure 1b) [39].
2.1. Co-Precipitation
2.2. Thermal and Hydrothermal Decomposition
2.3. Microemulsion and Inverse Micelles
2.4. Sol-Gel Processes
2.5. Biosynthesis
2.6. Functionalization of the Surface of NPs
3. Types of Antibiotics and Their Mechanism of Action
3.1. Tetracyclines
3.2. Penicillins
3.3. Sulphonamides
3.4. Macrolides
3.5. Fluoroquinolones
- First generation drugs (e.g., nalidixic acid)
- Second generation quinolones (e.g., ciprofloxacin) have increased gram-negative and systemic activity
- Third generation drugs (e.g., levofloxacin) have expanded activity against gram-positive bacteria and atypical pathogens
- Fourth generation quinolone drugs (currently only trovafloxacin) add significant activity against anaerobes
4. Analytical Methods for Antibiotic Detection and Quantification Based on MNPs
4.1. Electrochemical Sensors for Antibiotics
4.2. Optical Sensors for Antibiotics
- Wavelength-shift sensing, is used to monitor resonant wavelengths of light with the refractive index changes determined by the presence of analyte at the surface. The wavelengths of light cause the collective oscillation of valence electrons and subsequent absorption within the ultraviolet-visible (UV-Vis) band, due to interactions between the incident photons and the conduction band of a noble metal nanostructure.
- Surface-enhanced techniques, offer the detection of a target analyte by monitoring changes in the enhanced electromagnetic fields. Maximum enhancement is obtained when the LSPR wavelength is situated between the excitation wavelength and the wavelength of the scattered photon. This dependence of SERS on LSPR wavelength is complementary for molecular binding and identification studies.
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antibiotic Resistance. Available online: http://www.who.int/mediacentre/news/releases/2015/antibiotic-resistance/en (accessed on 16 March 2017).
- World Health Organization. Tackling Antibiotic Resistance from a Food Safety Perspective in Europe. 2011. Available online: http://www.euro.who.int/_data/assets/pdf_file/0005/136454/e94889 (accessed on 16 March 2017).
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 (accessed on 16 March 2017).
- European Medicines Agency—Veterinary Medicines Division. Sales of Veterinary Antimicrobial Agents in 26 EU/EEA Countries in 2013. Fifth ESVAC Report 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2015/10/WC500195687 (accessed on 16 March 2017).
- Seifrtova, M.; Novakova, L.; Lino, C.; Pena, A.; Solich, P. An Overview of Analytical Methodologies for the Determination of Antibiotics in Environmental Waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Herrera, A.V.; Ravelo-Pérez, L.M.; Hernández-Borges, L.; Afonso, M.M.; Palenzuela, J.A.; Rodríguez-Delgado, M.Á. Oxidized multi-walled carbon nanotubes for the dispersive solid-phase extraction of quinolone antibiotics from water samples using capillary electrophoresis and large volume sample stacking with polarity switching. J. Chromatogr. A 2011, 1218, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Tang, W.; Han, R.; Zhu, Y.; Wang, Q.; He, P.; Fang, Y. Sensitive analysis of aminoglycoside antibiotics via hyphenation of transient moving substitution boundary with field-enhanced sample injection in capillary electrophoresis. J. Chromatogr. A 2013, 1295, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lacina, P.; Mravcova, L.; Vavrova, M. Application of comprehensive two-dimensional gas chromatography with mass spectrometric detection for the analysis of selected drug residues in wastewater and surface water. J. Environ. Sci. 2013, 25, 204–212. [Google Scholar] [CrossRef]
- Migowska, N.; Caban, M.; Stepnowski, P.; Kumirska, J. Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Sci. Total Environ. 2012, 441, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lara, F.J.; Del Olmo-Iruela, M.; Cruces-Blanco, C.; Quesada-Molina, C.; Garcia-Campana, A.M. Advances in the determination of β-lactam antibiotics by liquid chromatography. TrAC Trends Anal. Chem. 2012, 38, 52–66. [Google Scholar] [CrossRef]
- Le Bizec, B.; Pinel, G.; Antignac, J.-P. Options for veterinary drug analysis using mass spectrometry. J. Chromatogr. A 2009, 1216, 8016–8034. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Mannelli, I.; Salvador, J.P.; Valera, E.; Marco, M.P. Biosensors for pharmaceuticals based on novel technology. TrAC Trends Anal. Chem. 2011, 30, 541–553. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Von Rechenberg, B.; Hofmann, H. Superparamagneticnanoparticles for biomedical applications. In Nanostructured Materials for Biomedical Applications; Tan, M.C., Ed.; Transworld Research Network: Kerala, India, 2009; pp. 119–149. ISBN 978-81-7895-397-7. [Google Scholar]
- Tadic, M.; Kralj, S.; Jagodic, M.; Hanzel, D.; Makovec, D. Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment. Appl. Surf. Sci. 2014, 322, 255–264. [Google Scholar] [CrossRef]
- Lu, A.-H.; Schmidt, W.; Matoussevitch, N.; Bönnemann, H.; Spliethoff, B.; Tesche, B.; Bill, E.; Kiefer, W.; Schüth, F. Nanoengineering of a Magnetically Separable Hydrogenation Catalyst. Angew. Chem. Int. Ed. Engl. 2004, 43, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Mornet, S.; Vasseur, S.; Grasset, F.; Verveka, P.; Goglio, G.; Demourgues, A.; Portier, J.; Pollert, E.; Duguet, E. Magnetic nanoparticle design for medical applications. Prog. Solid State Chem. 2006, 34, 237–247. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Taeghwan, H. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 8, 927–934. [Google Scholar] [CrossRef]
- Kavre, I.; Kostevc, G.; Kralj, S.; Vilfan, A.; Babič, D. Fabrication of magneto-responsive microgears based on magnetic nanoparticle embedded PDMS. RSC Adv. 2014, 4, 38316–38322. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.D.; Raj, B. Nanofluid with tunable thermal properties. Appl. Phys. Lett. 2006, 92, 043108. [Google Scholar] [CrossRef]
- He, L.; Wang, M.; Ge, J.; Yin, Y. Magnetic Assembly Route to Colloidal Responsive Photonic Nanostructures. Acc. Chem. Res. 2012, 45, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.; Kumar, T.J.; Kalyanasundaram, P.; Raj, B. Tunable Optical Filter. Meas. Sci. Technol. 2003, 14, 1289–1294. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Kulkarni, S.D.; Villar, P.S.; Smith, R.S.; Eberly, C.; Araneda, R.C.; Depireux, D.A.; Shapiro, B. Movement of magnetic nanoparticles in brain tissue: Mechanisms and safety. Nanomedicine 2015, 11, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.; Mahendran, V.; Felicia, L.J. A Simple, In-Expensive and Ultrasensitive Magnetic Nanofluid Based Sensor for Detection of Cations, Ethanol and Ammonia. J. Nanofluids 2013, 2, 112–119. [Google Scholar] [CrossRef]
- Mahendran, V. Nanofluid based optical sensor for rapid visual inspection of defects in ferromagnetic materials. Appl. Phys. Lett. 2012, 100, 073104. [Google Scholar] [CrossRef]
- Hosu, O.; Tertiş, M.; Săndulescu, R.; Cristea, C. Protein G Magnetic Beads Based ImmunosensorFor Sensitive Detection of Acetaminophen. Farmacia 2015, 63, 140–145. [Google Scholar]
- Florea, A.; Ravalli, A.; Cristea, C.; Sandulescu, R.; Marrazza, G. An optimized bioassay for Mucin1 detection in serum samples. Electroanalysis 2015, 27, 1594–1601. [Google Scholar] [CrossRef]
- Ravalli, A.; Marrazza, G.; Florea, A.; Cristea, C.; Sandulescu, R. Electrochemical Immunoassay for Mucin 1 Detection as a Diagnostic Tool in Ovarian Cancer. In Sensors and Microsystems. Lecture Notes in Electrical Engineering, Proceedings of the 17th National Conference, Brescia, Italy, 5–7 February 2013; Di Natale, C., Ferrari, V., Ponzoni, A., Sberveglieri, G., Ferrari, M., Eds.; Springer: Cham, Switzerland, 2014; Volume 268, pp. 165–169. ISBN 978-3-319-00684-0. [Google Scholar]
- Elliott, D.W.; Zhang, W. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structures, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-WCH: Weinheim, Germany, 2006; ISBN 978-3-527-60644-3. [Google Scholar]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Anchoring of Phosphonate and Phosphinate Coupling Molecules on Titania Particles. Chem. Mater. 2001, 13, 4367–4373. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nanochains and Nanobundles. ACS Nano 2015, 9, 9700–9707. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Makovec, D.; Čampelj, S.; Drofenik, M. Producing ultra-thin silica coatings on iron-oxide nanoparticles to improve their surface reactivity. J. Magn. Magn. Mater. 2010, 322, 1847–1853. [Google Scholar] [CrossRef]
- Kralj, S.; Rojnik, M.; Romih, R.; Jagodič, M.; Kos, J.; Makovec, D. Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles. J. Nanopart. Res. 2012, 14, 1151. [Google Scholar] [CrossRef]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently Functionalized Cobalt Nanoparticles as a Platform for Magnetic Separations in Organic Synthesis. Angew. Chem. Int. Ed. Engl. 2007, 46, 4909–4912. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Robert, N.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2014, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Isobe, T.; Senna, M. Magnetic properties of ultrafine magnetite particles and their slurries prepared via in-situ precipitation. Colloid Surf. A 1996, 109, 121–127. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Redl, F.X.; Black, C.T.; Papaefthymiou, G.C.; Sandstrom, R.L.; Yin, M.; Zeng, H.; Murray, C.B.; O’Brien, S.P. Magnetic, Electronic, and Structural Characterization of Nonstoichiometric Iron Oxides at the Nanoscale. J. Am. Chem. Soc. 2004, 126, 14583–14599. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.E.; Seip, C.T.; O’Connor, C.J. Magnetism of nanophase metal and metal alloy particles formed in ordered phases. J. Appl. Phys. 1999, 85, 5184. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. Engl. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse Micelle Synthesis and Characterization of Superparamagnetic MnFe2O4 Spinel Ferrite Nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Dong, W.T.; Zhu, C.S. Use of ethylene oxide in the sol-gel synthesis of α-Fe2O3 nanoparticles from Fe(III) salts. J. Mater. Chem. 2002, 12, 1676–1683. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.A.; Parikh, R.Y.; Baidakova, M.; Jouen, S.; Hannoyer, B.; Enoki, T.; Prasad, B.L.V.; Shouche, Y.S.; Ogale, S.; Sastry, M. Bacteria-mediated precursor-dependent biosynthesis of superparamagnetic iron oxide and iron sulfide nanoparticles. Langmuir 2008, 24, 5787–5794. [Google Scholar] [CrossRef] [PubMed]
- Pascal, C.; Pascal, J.L.; Favier, F.; Elidrissi Moubtassim, M.L.; Payen, C. Electrochemical Synthesis for the Control of γ-Fe2O3 Nanoparticle Size. Morphology, Microstructure, and Magnetic Behavior. Chem. Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Kahn, H.R.; Petrikowski, K. Anisotropic structural and magnetic properties of arrays of Fe26Ni74 nanowires electrodeposited in the pores of anodic alumina. J. Magn. Magn. Mater. 2000, 215–216, 526–528. [Google Scholar] [CrossRef]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioprocess Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Varanda, L.C.; JafelicciJúnior, M.; Beck Júnior, W. Magnetic and Multifunctional Magnetic Nanoparticles inNanomedicine: Challenges and Trends in Synthesis and Surface Engineering for Diagnostic and Therapy Applications in Biomedical Engineering. In Trends in Materials Science; Laskovski, A.N., Ed.; INTECH: Rijeka, Croatia, 2011; ISBN 978-953-307-513-6. [Google Scholar]
- Ur Rehman, M.S.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.I. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Kivirand, K.; Kagan, M.; Rinken, T. Biosensors for the Detection of Antibiotic Residues in Milk. In Nanotechnology and Nanomaterials: Biosensors—Micro and Nanoscale Applications; Rinken, T., Ed.; INTECH: Rijeka, Croatia, 2015; ISBN 978-953-51-2173-2. [Google Scholar]
- European Medicines Agency: Sales of Veterinary Antimicrobial Agents in 26 EU/EEA Countries in 2012. 4th ESVAC Report, 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/10/WC500175671 (accessed on 19 March 2017).
- Goel, S. Antibiotics in the Environment: A Review. In Emerging Micro-Pollutants in the Environment: Occurrence, Fate, and Distribution; Kurwadkar, S., Zhang, X., Ramirez, D., Mitchell, F.L., Eds.; ACS Symposium Series; Americam Chemical Society: Washington, DC, USA, 2015; Chapter 2; pp. 19–42. [Google Scholar] [CrossRef]
- Tetracycline: The American Society of Health-System Pharmacists. Available online: https://www.drugs.com/monograph/tetracycline.html (accessed on 23 March 2017).
- Zhao, H.; Wang, H.; Quan, X.; Tan, F. Amperometric sensor for tetracycline determination based on molecularly imprinted technique. Proc. Environ. Sci. 2013, 18, 249–257. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Pucci, M.J. (Eds.) Antibiotic Discovery and Development; Springer: New York, NY, USA, 2011; pp. 79–80. Available online: http://www.springer.com/gp/book/9781461413998 (accessed on 19 March 2016).
- Solensky, R. Hypersensitivity reactions to beta-lactam antibiotics. Clin. Rev. Allergy Immunol. 2003, 24, 201–220. [Google Scholar] [CrossRef]
- Gonzalez-Estrada, A.; Radojicic, C. Penicillin allergy: A practical guide for clinicians. Clevel. Clin. J. Med. 2015, 82, 295–300. [Google Scholar] [CrossRef]
- American Chemical Society Discovery and Development of Penicillin. Available online: https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html (accessed on 23 March 2017).
- Torok, E.; Moran, E.; Cooke, F. (Eds.) Oxford Handbook of Infectious Diseases and Microbiology; Oxford University Press: Oxford, UK, 2009; p. 56. ISBN 9780191039621. [Google Scholar]
- Actor, P.; Chow, A.W.; Dutko, F.J.; McKinlay, M.A. Chemotherapeutics, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germeny, 2005; Available online: http://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_173/full (accessed on 19 March 2017). [CrossRef]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The Mechanism of Action of Macrolides, Lincosamides and Streptogramin B Reveals the Nascent Peptide Exit Path in the Ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Andersson, M.I.; MacGowan, A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003, 51, 1–11. [Google Scholar] [CrossRef]
- King, D.E.; Malone, R.; Lilley, S.H. New Classification and Update on the Quinolone Antibiotics. Am. Fam. Physician 2000, 61, 2741–2748. [Google Scholar] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, L.E.; Movassaghi, K.; Bahrami, F.; Enayati, M.; Chianese, A. Determination of Drugs in Biological Sample by Using Modified Magnetic Nanoparticles and HPLC. Chem. Eng. Trans. 2014, 38, 391–396. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Liu, Y.; Ma, J. Development of magnetic molecularly imprinted polymers with double templates for the rapid and selective determination of amphenicol antibiotics in water, blood, and egg samples. J. Chromatogr. A 2016, 1473, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Sczesny, S.; Hoper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.; Farber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernández-Borges, J.; Afonso, M.M.; Palenzuela, J.A.; Rodríguez-Delgado, M.Á. Comparison between magnetic and non magnetic multi-walled carbon nanotubes-dispersive solid-phase extraction combined with ultra-high performance liquid chromatography for the determination of sulfonamide antibiotics in water samples. Talanta 2013, 116, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, S.; Hu, Y.; Li, Z.; Luo, F.; He, Z. Electrochemical Immunosensor Based on the Chitosan-Magnetic Nanoparticles for Detection of Tetracycline. Food Anal. Methods 2016, 9, 2972–2978. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, W.; Pei, M.; Ding, F. GR-Fe3O4NPs and PEDOT-AuNPs composite based electrochemical aptasensor for the sensitive detection of penicillin. Anal. Methods 2016, 22, 4391–4397. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhang, H.R.; Yan, Z.D.; Li, T.H.; Chen, Y.J.; Xu, Q.; Jiang, Q.L. Electrochemical simultaneous assay of chloramphenicol and PCB72 using magnetic and aptamer-modified quantum dot-encoded dendritic nanotracers for signal amplification. Microchim. Acta 2016, 183, 1099–1106. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Shi, Z.; Duan, N.; Fang, C.; Dai, S.; Wang, Z. Aptamer-based fluorescence biosensor for chloramphenicol determination using up conversion nanoparticles. Food Control. 2015, 50, 597–604. [Google Scholar] [CrossRef]
- Hao, L.L.; Duan, N.; Wu, S.J.; Xu, B.C.; Wang, Z.P. Chemiluminescent aptasensor for chloramphenicol based on N-(4-aminobutyl)-N-ethylisoluminol-functionalized flower-like gold nanostructures and magnetic nanoparticles. Anal. Bioanal. Chem. 2015, 407, 7907–7915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Dai, H.; Ni, P.; Jiang, S.; Lu, W.; Li, Z.; Li, Z. A colorimetric biosensor using Fe3O4 nanoparticles for highly sensitive and selective detection of tetracyclines. Sens. Actuators B Chem. 2016, 236, 621–626. [Google Scholar] [CrossRef]
- Hulanicki, A.; Geab, S.; Ingman, F. Chemical sensors Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Cristea, C.; Florea, A.; Tertis, M.; Săndulescu, R. Immunosensors. In Biosensors-Micro and Nanoscale Aplications; Rinken, T., Ed.; INTECH: Rijeka, Croatia, 2015; pp. 165–202. ISBN 978-953-51-2173-2. Available online: http://www.intechopen.com/books/biosensors-micro-and-nanoscale-applications/immunosensors (accessed on 19 March 2016).
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors-principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical detection of protein using a double aptamer sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wang, E.K. Electrochemical biosensors based on magnetic micro/nano particles. Electrochim. Acta 2012, 84, 62–73. [Google Scholar] [CrossRef]
- Valera, E.; Muriano, A.; Pividori, I.; Sanchez-Baeza, F.; Marco, M.-P. Development of a Coulombimetric immunosensor based on specific antibodies labeled with CdS nanoparticles for sulfonamide antibiotic residues analysis and its application to honey samples. Biosens. Bioelectron. 2013, 43, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJfexonuclease assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Asadpour-Zeynali, K.; Mollarasouli, F. Novel electrochemical biosensor based on PVP capped CoFe2O4@CdSen core-shell nanoparticles modified electrode for ultra-trace level determination of rifampicin by square wave adsorptive stripping voltammetry. Biosens. Bioelectron. 2017, 92, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, S.; Benvidi, A. Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@oleic acid nanoparticle electrodes for tetracycline detection. Biosens. Bioelectron. 2016, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chadra, P.; Song, K.-M.; Ban, C.; Shim, Y.-B. Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens. Bioelectron. 2012, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.; Ma, H.; Zhang, Y. Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Pundir, C.S. Optimal strategies for determination of free/extractable and total microcystins in lake sediment. Anal. Chim. Acta 2011, 701, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Karimi, M. Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 2017, 167, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhang, Z.; Yang, Z.; Zeng, J.; Jiang, Y. Imprinted electrochemical sensor based on magnetic multi-walled carbon nanotube for sensitive determination of kanamycin. J. Electroanal. Chem. 2015, 755, 7–14. [Google Scholar] [CrossRef]
- Mungroo, N.A.; Neethirajan, S. Biosensors for the Detection of Antibiotics in PoultryIndustr—A Review. Biosensors 2014, 4, 472–493. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.-Y.; Chang, Y.-F.; Ng, M.-Y.; Liu, W.-C.; Lin, C.-H.; Wu, H.-T.; Chou, C. Localized Surface Plasmon Coupled Fluorescence Fiber-Optic Biosensor with Gold Nanoparticles. Anal. Chem. 2007, 79, 3487–3493. [Google Scholar] [CrossRef] [PubMed]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable microfluidic integrated plasmonic platform for pathogen detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kerman, K.; Nagatani, N.; Takamura, Y.; Tamiya, E. Label-free detection of peptide nucleic acid-DNA hybridization using localized surface plasmon resonance based optical biosensor. Anal. Chem. 2005, 77, 6976–6984. [Google Scholar] [CrossRef]
- Endo, T.; Kerman, K.; Nagatani, N.; Hiepa, H.M.; Kim, D.K.; Yonezawa, Y.; Nakano, K.; Tamiya, E. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structured nanoparticle layer nanochip. Anal. Chem. 2006, 78, 6465–6475. [Google Scholar] [CrossRef] [PubMed]
- Kawazumi, H.; Gobi, K.V.; Ogino, K.; Maeda, H.; Miura, N. Compact surface plasmon resonance (SPR) immunosensor using multichannel for simultaneous detection of small molecule compounds. Sens. Actuators B Chem. 2005, 108, 791–796. [Google Scholar] [CrossRef]
- Chinowsky, T.M.; Soelberg, S.D.; Baker, P.; Swanson, N.R.; Kauffman, P.; Mactutis, A.; Grow, M.S.; Atmar, R.; Yee, S.S.; Furlong, C.E. Portable 24-analyte surface plasmon resonance instruments for rapid, versatile biodetection. Biosens. Bioelectron. 2007, 22, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Zou, S.; Schatz, G.C.; van Duyne, R.P. Nanoscale optical biosensor: Short range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Z.; Ni, W.; Woo, K.C.; Lin, H.Q.; Sun, L.; Yan, C.; Wang, J. Plasmon coupling in clusters composed of two-dimensionally ordered gold nanocubes. Small 2009, 5, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; van Duyne, R.P. A unified view of propagating and localized surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2004, 379, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, W. Surface-enhanced Raman scattering. J. Phys. Condens. Matter 1992, 4, 1143–1212. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Gersten, J.; Nitzan, A. Electromagnetic theory of enhanced Raman scattering by molecules adsorbed on rough surfaces. J. Chem. Phys. 1980, 73, 3023–3037. [Google Scholar] [CrossRef]
- Metiu, H.; Das, P. The electromagnetic theory of surface enhanced spectroscopy. Annu. Rev. Phys. Chem. 1984, 35, 507–536. [Google Scholar] [CrossRef]
- Kao, J. Comparison of Surface Plasmon Resonance and Localized Surface Plasmon Resonance-based optical fibre sensors. J. Phys. Conf. Ser. 2011, 307, 012050. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Galatus, R.; Conte, L.; Zeni, L. Performance comparison of two sensors based on surface plasmon resonance in a plastic optical fiber. Sensors 2013, 13, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; van Duyne, R.P. Localized surface plasmon resonance biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Qui, L.; Larson, T.A.; Smith, D.; Vitkin, E.; Modell, M.D.; Korgel, B.A.; Sokolov, K.V.; Hanlon, E.B.; Itzkan, I.; Perelman, L.T. Observation of plasmon line broadening in single gold nanorods. Appl. Phys. Lett. 2008, 93, 153106. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.Z.; Li, R.S.; Huang, C.Z. Localized surface plasmon resonance of gold nanorods and assemblies in the view of biomedical analysis. TrAC Trends Anal. Chem. 2016, 80, 429–443. [Google Scholar] [CrossRef]
- Liao, W.S.; Chen, X.; Yang, T.; Castellana, E.T.; Chen, J.; Cremer, P.S. Benchtop chemistry for the rapid prototyping of label-free biosensors: Transmission localized surface plasmon resonance platforms. Biointerphases 2009, 4, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kerman, K.; Saito, M.; Sathuluri, R.R.; Endo, T.; Yamamura, S.; Kwon, Y.S.; Tamiya, E. Label-free DNA biosensor based on localized surface plasmon resonance coupled with interferometry. Anal. Chem. 2007, 79, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Novo, C.; Funston, A.; Wang, H.; Staleva, H.; Zou, S.; Mulvaney, P.; Xia, Y.; Hartland, G.V. Dark-field microscopy studies of single metal nanoparticles: Understanding the factors that influence the line width of the localized surface plasmon resonance. J. Mater. Chem. 2008, 18, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, D.J.; Kim, D. Effect of silver nanoisland-embedded grating for surface plasmon based total internal reflection fluorescence imaging. Proc. SPIE 2009, 7224, 722417. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fiorini, M.T.; Abell, C.; Williams, D.H. Binding of vancomycin group antibiotics to D-alanine and D-lactate presenting self-assembled monolayers. Bioorgan. Med. Chem. 2000, 8, 2609–2616. [Google Scholar] [CrossRef]
- Rao, J.; Yan, L.; Xu, B.; Whitesides, G.M. Using surface plasmon resonance to study the binding of vancomycin and its dimer to self-assembled monolayers presenting D-Ala-D-Ala. J. Am. Chem. Soc. 1999, 121, 2629–2630. [Google Scholar] [CrossRef]
- Ferguson, J.; Baxter, A.; Young, P.; Kennedy, G.; Elliott, C.; Weigel, S.; Gatermann, R.; Ashwind, H.; Stead, S.; Sharman, M. Detection of chloramphenicol and chloramphenicol glucuronide residues in poultry muscle, honey, prawn and milk using a surface plasmon resonance biosensor and Qflex((R)) kit chloramphenicol. Anal. Chim. Acta 2005, 529, 109–113. [Google Scholar] [CrossRef]
- Fernández, F.; Pinacho, D.G.; Sáncez-Baeza, F.; Marco, M.P. Portable Surface Plasmon Resonance Immunosensor for the Detection of Fluoroquinolone Antibiotic Residues in Milk. J. Agric. Food Chem. 2011, 59, 5036–5043. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q. Surface plasmon resonance sensor for antibiotics detection based on photoinitiatedpolymerization molecularly imprinted array. Talanta 2016, 161, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Conta, G.; Campanella, L.; Favero, G.; Sanzò, G.; Mazzei, F.; Antiochia, R. A Flow SPR Immunosensor Based on a Sandwich Direct Method. Biosensors 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qu, C.; Kuang, H.; Xu, L.; Liu, L.; Hua, Y.; Wang, L.; Xu, C. Simple, rapid and sensitive detection of antibiotics based on the side-by-side assembly of gold nanorod probes. Biosens. Bioelectron. 2011, 26, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ko, J.; Lim, H.B. Application of magnetic and core-shell nanoparticles to determine enrofloxacin and its metabolite using laser induced fluorescence microscope. Anal. Chim. Acta 2013, 771, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Font, H.C.; Diserens, J.-M.; Sanchez-Baeza, F.; Marco, M.P. Generation of broad specificity antibodies for sulfonamide antibiotics and development of an enzyme-linked immunosorbent assay (ELISA) for the analysis of milk samples. J. Agric. Food Chem. 2009, 57, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Pasche, S.; Pinacho, D.G.; Font, H.; Diserens, J.-M.; Sanchez-Baeza, F.; Granier, B.; Voirin, G.; Marco, M.P. Wavelength-interrogated optical biosensor for multi-analyte screening of sulfonamide, fluoroquinolone, b-lactam and tetracycline antibiotics in milk. TrAC Trends Anal. Chem. 2009, 28, 769–777. [Google Scholar] [CrossRef]
- Kelkar, H.; Wang, D.; Martín-Cano, D.; Hoffmann, B.; Christiansen, S.; Götzinger, S.; Sandoghdar, V. Sensing Nanoparticles with a Cantilever-Based Scannable Optical Cavity of Low Finesse and Sub-λ3 Volume. Phys. Rev. Appl. 2015, 4, 054010. [Google Scholar] [CrossRef]
- Datar, R.; Kim, S.; Jeon, S.; Hesketh, P.; Manalis, S.; Boisen, A.; Thundat, T. Cantilever Sensors: Nanomechanical Tools for Diagnostics. MRS Bull. 2009, 34, 449–454. [Google Scholar] [CrossRef]
- Gfeller, K.Y.; Nugaeva, N.; Hegner, M. Rapid Biosensor for Detection of Antibiotic-Selective Growth of Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, L.G.; Moreno, M.; Alvarez, M.; Lechuga, L.M. Nanomechanical biosensors: A new sensing tool. TrAC Trends Anal. Chem. 2006, 25, 196–206. [Google Scholar] [CrossRef]
- Chan, P.-H.; Liu, H.-B.; Chen, Y.W.; Chan, K.-C.; Tsang, C.-W.; Leung, Y.-C.; Wong, K.-Y. Rational design of a novel fluorescent biosensor for beta-lactam antibiotics from a class A beta-lactamase. J. Am. Chem. Soc. 2004, 126, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-B.; Gan, N.; Ren, H.-X.; Li, T.; Cao, Y.; Hu, F.; Chen, Y. Switch-on fluorescence scheme for antibiotics based on a magnetic composite probe with aptamer and hemin/G-quadruplex coimmobilized nano-Pt-luminol as signal tracer. Talanta 2013, 147, 296–301. [Google Scholar] [CrossRef] [PubMed]





| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Co-precipitation |
|
|
| Thermal and hydrothermal decomposition |
|
|
| Microemulsion and inverse micelles |
|
|
| Sol-gel processes |
|
|
| Biosynthesis |
|
|
| Electrochemical methods |
|
|
| Flow injection synthesis |
|
|
| Aerosol/Vapor methods (spray and laser pyrolysis) |
| |
| Coatings and functionalization at the surface of NPs |
|
|
| Antibiotics | Applications | Side Effects |
|---|---|---|
| Penicillins |
|
|
| Tetracyclines |
|
|
| Sulfonamides |
|
|
| Macrolides |
|
|
| Fluoroquinolones |
|
|
| Aminoglycosides |
|
|
| Phenicol |
|
|
| Licosamides |
|
|
| Method/Application | Analytical Parameters | Observations | Reference |
|---|---|---|---|
| Liquid chromatography (LC)-based coupled methods | |||
| HPLC-UV/Determination of ceftriaxone in human plasma (HPLC-UV—high performance liquid chromatography with UV detector; LR—linear range; LOD—lowest limit of detection) | LR: 0.06–40 µg mL−1 LOD: 20 ng mL−1 | Ag modified- magnetic nanoparticle (Ag-MNPs) were used for the preconcentration of ceftriaxone, an enrichment factor of 19 being obtained in optimal conditions | [72] |
| HPLC-UV/Determination of chloramphenicol (CAP), florfenicol (FF) and thiamphenicol (TAP) in water, chicken blood and egg samples | LOD: CAP: 0.16 µg kg−1 FF: 0.08 µg kg−1 TAP: 0.08 µg kg−1 Recoveries from 88.3% to 99.1% | A magnetic mesoporous dual-template molecularly imprinted polymer (Fe3O4@mSiO2@DMIP) was synthetised;-The obtained Fe3O4@mSiO2@DMIP particles were applied as a magnetic solid-phase extraction sorbent for the rapid and selective extraction of CAP, FF, and TAP | [73] |
| LC-MS/MS/Determination of tetracycline, chlortetracycline, oxytetracycline and tyosin in a field fertilized with liquid manure (LC-MS/MS—LC with tandem mass spectrometry) | Amount detected: Tetracycline from 86.2 to 4000 µg kg−1 Chlortetracycline from 4.6 to 100 µg kg−1 Oxytetracycline not detected Tyosin not detected | Tetracyclines were detected in the environment, are persistent residues, and accumulate in soil | [74] |
| HPLC-MS/MS/Determination of erythromycin, tylosin and other antibiotics from surface waters, soil and liquid manures | Amount detected:Erythromycin in water 0.3 µg L−1 Sulfadiimide in soil 1000-2000 µg kg−1 | Test were performed seven months after application, this indicates the high stability of some antibiotics in manure and soil | [75] |
| UHPL-DAD/Determination of 11 sulfonamide antibiotics in mineral waters with different mineral content (UHPL-DAD—ultra-high pressure liquid chromatography-diode array Detection) | LODs lower than 32 pg mL−1 for all of the analyzed compounds | Pristine multi-walled carbon nanotubes (MWCNTs) and magnetic-MWCNTs (m-MWCNTs) were used as sorbents for off-line dispersive solid-phase extraction (dSPE) of antibiotics from mineral waters | [76] |
| Electrochemical sensors | |||
| EIS/Determination of tetracycline in milk | LR: 0.08–1 ng mL−1 LOD: 0.032 ng mL−1 | Electrochemical immunosensor based on gold electrode-modified carboxyl-Fe3O4 nanoparticle (MNPs) by chitosan (CS) as linker | [77] |
| EIS/Determination of penicillin in milk | LR: 0.1–200 ng mL−1 LOD: 0.057 ng mL−1 | Electrochemical aptasensor based on GCE modified with poly(3,4-ethylenedioxythiophene)–gold nanoparticles composite (PEDOT–AuNPs) and magnetic graphene nanocomposite (GR–Fe3O4NPs) | [78] |
| Voltammetry/Determination of chloramphenicol in fish samples | LR: 0.001–100 ng mL−1 LOD: 0.33 pg mL−1 | Voltammetric aptasensor based on magnetic gold nanoparticles (Fe3O4@Au) and a dendritic polymerase used in order to link these nanocomposite to quantum dots (CdS or PbS) and to form the nanotracers | [79] |
| Optical sensors | |||
| Fluorescence biosensor/Determination of chloramphenicol in milk | LR: 0.01–1 ng mL−1LOD: 0.01 ng mL−1 | Aptasensor based on aptamer-conjugated magnetic nanoparticles (MNPs) | [80] |
| Chemiluminescent biosensor/Determination of chloramphenicol in milk | LR: 0.01–0.20 ng mL−1 LOD: 0.01 ng mL−1 | Aptasensor based onmagnetic nanoparticles (MNPs) and thiolated hybridized complementary strand modified N-(4-aminobutyl)-N-ethylisoluminol (ABEI)-functionalized flower-like gold nanostructures (AuNFs) | [81] |
| Colorimetric biosensor/Determination of oxytetracycline (OTC), tetracycline (TC) and doxycyline (DOX) | OTC: LR: 50–1000 nM LOD: 26 nM TC: LR: 100–1000 nMLOD: 45 nM DOX: LR: 50–1000 nM LOD: 48 nM | Enzyme sensor based on Fe3O4 magnetic nanoparticles (Fe3O4 MNPs) The elaborated biosensor was applied for the determination of DOX content in drugs | [82] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, C.; Tertis, M.; Galatus, R. Magnetic Nanoparticles for Antibiotics Detection. Nanomaterials 2017, 7, 119. https://doi.org/10.3390/nano7060119
Cristea C, Tertis M, Galatus R. Magnetic Nanoparticles for Antibiotics Detection. Nanomaterials. 2017; 7(6):119. https://doi.org/10.3390/nano7060119
Chicago/Turabian StyleCristea, Cecilia, Mihaela Tertis, and Ramona Galatus. 2017. "Magnetic Nanoparticles for Antibiotics Detection" Nanomaterials 7, no. 6: 119. https://doi.org/10.3390/nano7060119
APA StyleCristea, C., Tertis, M., & Galatus, R. (2017). Magnetic Nanoparticles for Antibiotics Detection. Nanomaterials, 7(6), 119. https://doi.org/10.3390/nano7060119