Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation
Abstract
:1. Introduction
2. Results and Discussion
2.1. AgNP Films Were Inkjet-Printed onto Active Papers and Reference Papers
2.2. Resistivity Measurements Showed Sintering Inhibition of AgNP Films on Active Papers
2.3. Chloride Migrated from the Coating into the AgNP Film
2.4. Chloride Impaired the Colloidal Stability of the AgNP Ink
2.5. Chloride Had Opposing Effects on Room-Temperature Sintering: Pretreatment vs. Post-Treatment
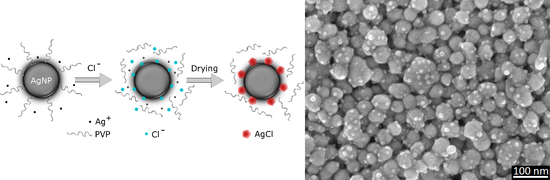
2.6. AgCl Nanocrystal Formation Is THE Main Sintering Inhibition Mechanism
3. Materials and Methods
3.1. Comparison Substrates and Substrate Pretreatments
3.2. Inks and Printing
3.3. Sintering
3.4. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Öhlund, T.; Örtegren, J.; Forsberg, S.; Nilsson, H.E. Paper surfaces for metal nanoparticle inkjet printing. Appl. Surf. Sci. 2012, 259, 731–739. [Google Scholar] [CrossRef]
- Öhlund, T.; Andersson, M. Effect of Paper Properties on Electrical Conductivity and Pattern Definition for Silver Nanoparticle Inkjet Ink. In Proceedings of the LOPE-C 2012, Munich, Germany, 19–21 June 2012; pp. 115–119. [Google Scholar]
- Öhlund, T.; Örtegren, J.; Andersson, H.; Nilsson, H.-E. Sintering Methods for Metal Nanoparticle Inks on Flexible Substrates. In Proceedings of the NIP & Digital Fabrication Conference (NIP 25), Louisville, KY, USA, 20–24 September 2009; pp. 614–617. [Google Scholar]
- Wunscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Niittynen, J.; Abbel, R.; Mäntysalo, M.; Perelaer, J.; Schubert, U.S.; Lupo, D. Alternative sintering methods compared to conventional thermal sintering for inkjet printed silver nanoparticle ink. Thin Solid Films 2014, 556, 452–459. [Google Scholar] [CrossRef]
- Kim, H.-S.; Dhage, S.; Shim, D.-E.; Hahn, H.T. Intense pulsed light sintering of copper nanoink for printed electronics. Appl. Phys. A 2009, 97, 791–798. [Google Scholar] [CrossRef]
- Öhlund, T.; Schuppert, A.K.; Hummelgård, M.; Bäckström, J.; Nilsson, H.-E.; Olin, H. Inkjet Fabrication of Copper Patterns for Flexible Electronics: Using Paper with Active Precoatings. ACS Appl. Mater. Interfaces 2015, 7, 18273–18282. [Google Scholar] [CrossRef] [PubMed]
- Abbel, R.; van Lammeren, T.; Hendriks, R.; Ploegmakers, J.; Rubingh, E.J.; Meinders, E.R.; Groen, W.A. Photonic flash sintering of silver nanoparticle inks: A fast and convenient method for the preparation of highly conductive structures on foil. MRS Commun. 2012, 2, 145–150. [Google Scholar] [CrossRef]
- Wakuda, D.; Hatamura, M.; Suganuma, K. Novel method for room temperature sintering of Ag nanoparticle paste in air. Chem. Phys. Lett. 2007, 441, 305–308. [Google Scholar] [CrossRef]
- Layani, M.; Grouchko, M.; Shemesh, S.; Magdassi, S. Conductive patterns on plastic substrates by sequential inkjet printing of silver nanoparticles and electrolyte sintering solutions. J. Mater. Chem. 2012, 22, 14349–14352. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Mihailescu, C.F.; Anghel, D.F.; Magdassi, S. Conductive Inks with a “Built-In” Mechanism That Enables Sintering at Room Temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Magdassi, S.; Grouchko, M.; Berezin, O.; Kamyshny, A. Triggering the Sintering of Silver Nanoparticles at Room Temperature. ACS Nano 2010, 4, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, T.; Schuppert, A.; Andres, B.; Andersson, H.; Forsberg, S.; Schmidt, W.; Nilsson, H.-E.; Andersson, M.; Zhang, R.; Olin, H. Assisted sintering of silver nanoparticle inkjet ink on paper with active coatings. RSC Adv. 2015, 5, 64841–64849. [Google Scholar] [CrossRef]
- Andersson, H.; Manuilskiy, A.; Lidenmark, C.; Gao, J.; Öhlund, T.; Forsberg, S.; Örtegren, J.; Schmidt, W.; Nilsson, H.-E. The influence of paper coating content on room temperature sintering of silver nanoparticle ink. Nanotechnology 2013, 24, 455203. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, J.; Leppaniemi, J.; Mattila, T.; Eiroma, K. Sintering of inkjet printed silver tracks with boiling salt water. J. Mater. Chem. C 2014, 2, 3577–3582. [Google Scholar] [CrossRef]
- Chiu, C.-K.; Choi, Y.-J.; Luo, T.-J.M. Formation of AgCl Cubic Crystals Induced by Shrinkage of Sol-Gel Silica Film. Cryst. Growth Des. 2012, 12, 4727–4732. [Google Scholar] [CrossRef]
- Anderson, R.; Buscall, R.; Eldridge, R.; Mulvaney, P.; Scales, P.J. Ostwald ripening of comb polymer stabilised Ag salt nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 459, 58–64. [Google Scholar] [CrossRef]
- Huang, H.H.; Ni, X.P.; Loy, G.L.; Chew, C.H.; Tan, K.L.; Loh, F.C.; Deng, J.F.; Xu, G.Q. Photochemical Formation of Silver Nanoparticles in Poly(N-vinylpyrrolidone). Langmuir 1996, 12, 909–912. [Google Scholar] [CrossRef]
- Bonet, F.; Tekaia-Elhsissen, K.; Sarathy, K.V. Study of interaction of ethylene glycol/PVP phase on noble metal powders prepared by polyol process. Bull. Mater. Sci. 2000, 23, 165–168. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, P.; Liu, D.F.; Yuan, H.J.; Yan, X.Q.; Zhou, Z.P.; Wang, J.X.; Song, L.; Liu, L.F.; Zhou, W.Y.; et al. Evidence for the Monolayer Assembly of Poly(vinylpyrrolidone) on the Surfaces of Silver Nanowires. J. Phys. Chem. B 2004, 108, 12877–12881. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, B.; Hu, L. PVP Protective Mechanism of Ultrafine Silver Powder Synthesized by Chemical Reduction Processes. J. Solid State Chem. 1996, 121, 105–110. [Google Scholar] [CrossRef]
- Dong, L.; Liang, D.; Gong, R. In Situ Photoactivated AgCl/Ag Nanocomposites with Enhanced Visible Light Photocatalytic and Antibacterial Activity. Eur. J. Inorg. Chem. 2012, 2012, 3200–3208. [Google Scholar] [CrossRef]










| Ink | Capping Agent | Solvent | Resistivity(RBS) 1 |
|---|---|---|---|
| Main | PVP | TGEE | 106 |
| Var1 | PVP | Ethanol/EG 3 | 105 |
| Var2 | Other 2 | Ethanol/EG 3 | <100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öhlund, T.; Hummelgård, M.; Olin, H. Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation. Nanomaterials 2017, 7, 224. https://doi.org/10.3390/nano7080224
Öhlund T, Hummelgård M, Olin H. Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation. Nanomaterials. 2017; 7(8):224. https://doi.org/10.3390/nano7080224
Chicago/Turabian StyleÖhlund, Thomas, Magnus Hummelgård, and Håkan Olin. 2017. "Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation" Nanomaterials 7, no. 8: 224. https://doi.org/10.3390/nano7080224
APA StyleÖhlund, T., Hummelgård, M., & Olin, H. (2017). Sintering Inhibition of Silver Nanoparticle Films via AgCl Nanocrystal Formation. Nanomaterials, 7(8), 224. https://doi.org/10.3390/nano7080224