Synthesis of Au-Pd Bimetallic Nanoflowers for Catalytic Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Au-Pd Nanoflowers
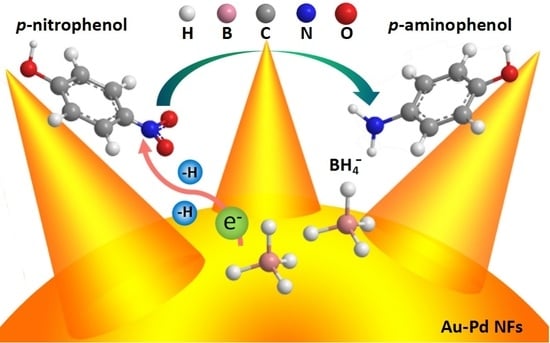
2.2. Catalytic Reduction of 4-Nitrophenol
3. Materials and Methods
3.1. Synthesis of Au-Pd NFs(Au Core and Au1Pd1 Core)
3.2. Synthesis of Au@Pd NFs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Haruta, M. Chance and necessity: My encounter with gold catalysts. Angew. Chem. Int. Ed. 2014, 53, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, H. Novel Metal nanomaterials and their catalytic applications. Molecules 2015, 20, 17070–17092. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Du, J.S.; Gilroy, K.D.; Yang, D.; Xia, Y.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Goodman, D.W. Pd-Au bimetallic catalysts: Understanding alloy effects from planar models and (supported) nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.G.; Kunz, M.R.; Skrabalak, S.E. Seeding a new kind of garden: Synthesis of architecturally defined multimetallic nanostructures by seed-mediated co-reduction. Acc. Chem. Res. 2015, 48, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.; Kalevaru, V.N.; Martin, A. Bimetallic catalysts containing gold and palladium for environmentally important reactions. Catalysts 2016, 6, 97. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Nanostars shine bright for you: Colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Narayanan, R.; El-Sayed, M.A. Enhancing colloidal metallic nanocatalysis: Sharp edges and corners for solid nanoparticles and cage effect for hollow ones. Acc. Chem. Res. 2013, 46, 1795–1805. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Garlyyev, B.; El-Sayed, M.A. Controlling the catalytic efficiency on the surface of hollow gold nanoparticles by introducing an inner thin layer of platinum or palladium. J. Phys. Chem. Lett. 2014, 5, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Soetan, N.; Zarick, H.F.; Banks, C.; Webb, J.A.; Libson, G.; Coppola, A.; Bardhan, R. Morphology-directed catalysis with branched gold nanoantennas. J. Phys. Chem. C 2016, 120, 10320–10327. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef]
- Downing, R.S.; Kunkeler, P.J.; Bekkum, H.V. Catalytic syntheses of aromatic amines. Catal. Today 1997, 37, 121–136. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, M.; Kim, Y.; Kang, S.W.; Lee, J.H.; Han, S.W. Synthesis and electrocatalytic activity of Au-Pd alloy nanodendrites for ethanol oxidation. J. Phys. Chem. C 2010, 114, 7689–7693. [Google Scholar] [CrossRef]
- Han, Y.; Yang, X.; Liu, Y.; Ai, Q.; Liu, S.; Sun, C.; Liang, F. Supramolecular controlled cargo release via near infrared tunable cucurbit[7]uril-gold nanostars. Sci. Rep. 2016, 6, 22239. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Kobayashi, H.; Yu, T.; Wang, J.; Kim, M.J.; Li, Z.Y.; Rycenga, M.; Xia, Y. Synthesis of Pd-Au bimetallic nanocrystals via controlled overgrowth. J. Am. Chem. Soc. 2010, 132, 2506–2507. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, L.; Jiao, C.; Huang, Z.; Liang, F.; Liu, S.; Wang, Y.; Zhang, H. Preparation of Rh/Ni bimetallic nanoparticles and their catalytic activities for hydrogen generation from hydrolysis of KBH4. Catalysts 2017, 7, 125. [Google Scholar] [CrossRef]
- Zhu, X.; Jia, H.; Zhu, X.M.; Cheng, S.; Zhuo, X.; Qin, F.; Yang, Z.; Wang, J. Selective Pd deposition on Au nanobipyramids and Pd site-dependent plasmonic photocatalytic activity. Adv. Funct. Mater. 2017, 27, 1700016. [Google Scholar] [CrossRef]
- Srisombat, L.; Nonkumwong, J.; Suwannarat, K.; Kuntalue, B.; Ananta, S. Simple preparation Au/Pd core/shell nanoparticles for 4-nitrophenol reduction. Colloid Surf. A Physicochem. Eng. Asp. 2017, 512, 17–25. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Jin, Y.; Lei, Y.; Johnston, R.L. One-pot synthesis of dealloyed AuNi nanodendrite as a bifunctional electrocatalyst for oxygen reduction and borohydride oxidation reaction. Adv. Funct. Mater. 2017, 27, 1700260. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Ma, H.; Zhang, J. Electrochemical synthesis, voltammetric behavior, and electrocatalytic activity of Pd nanoparticles. J. Phys. Chem. C 2008, 112, 2456–2461. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, Y.; Luo, X.; Li, J.; Zhang, H.; Yang, B. One-pot, seedless synthesis of flowerlike Au–Pd bimetallic nanoparticles with core-shell-like structure via sodium citrate coreduction of metal ions. CrystEngComm 2012, 14, 7036–7042. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, C.; Huang, H.; Zhang, L.; Jiang, Z.; Chen, G.; Jia, Y.; Kuang, Q.; Xie, Z.; Zheng, L. Surfactant-concentration-dependent shape evolution of Au-Pd alloy nanocrystals from rhombic dodecahedron to trisoctahedron and hexoctahedron. Small 2013, 9, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zeng, J.; Tao, J.; Johnson, M.C.; Schmidt-Krey, I.; Blubaugh, L.; Zhu, Y.; Gu, Z.; Xia, Y. Kinetically controlled overgrowth of Ag or Au on Pd nanocrystal seeds: From hybrid dimers to nonconcentric and concentric bimetallic nanocrystals. J. Am. Chem. Soc. 2012, 134, 15822–15831. [Google Scholar] [CrossRef] [PubMed]
- Óvári, L.; Bugyi, L.; Majzik, Z.; Berkó, A.; Kiss, J. Surface structure and composition of Au-Rh bimetallic nanoclusters on TiO2(110): A LEIS and STM study. J. Phys. Chem. C 2008, 112, 18011–18016. [Google Scholar] [CrossRef]
- Óvári, L.; Berkó, A.; Balázs, N.; Majzik, Z.; Kiss, J. Formation of Rh-Au core-shell nanoparticles on TiO2(110) surface studied by STM and LEIS. Langmuir 2010, 26, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Luo, K.; Wei, T.; Yan, Z.; Kumar, D.; Yi, C.W.; Goodman, D.W. The nature of the active site for vinyl acetate synthesis over Pd-Au. Catal. Today 2006, 117, 37–45. [Google Scholar] [CrossRef]
- Yudanov, I.V.; Neyman, K.M. Stabilization of Au at edges of bimetallic PdAu nanocrystallites. Phys. Chem. Chem. Phys. 2010, 12, 5094–5100. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Bingwa, N.; Patala, R.; Noh, J.H.; Ndolomingo, M.J.; Tetyana, S.; Bewana, S.; Meijboom, R. Synergistic effects of gold-palladium nanoalloys and reducible supports on the catalytic reduction of 4-nitrophenol. Langmuir 2017, 33, 7086–7095. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, Y.; Goodman, D.W. Reaction kinetics and Polarization-Modulation Infrared Reflection Absorption Spectroscopy (PM-IRAS) investigation of CO oxidation over supported Pd-Au alloy catalysts. J. Phys. Chem. C 2010, 114, 4036–4043. [Google Scholar] [CrossRef]
- Kiss, J.; Óvári, L.; Oszkó, A.; Pótári, G.; Tóth, M.; Baán, K.; Erdóhelyi, A. Structure and reactivity of Au-Rh bimetallic clusters on titanate nanowires, nanotubes and TiO2(110). Catal. Today 2012, 181, 163–170. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Liang, F.; Chen, R.; Liu, S.; Zhang, H. Synthesis of Au-Pd Bimetallic Nanoflowers for Catalytic Reduction of 4-Nitrophenol. Nanomaterials 2017, 7, 239. https://doi.org/10.3390/nano7090239
Ma T, Liang F, Chen R, Liu S, Zhang H. Synthesis of Au-Pd Bimetallic Nanoflowers for Catalytic Reduction of 4-Nitrophenol. Nanomaterials. 2017; 7(9):239. https://doi.org/10.3390/nano7090239
Chicago/Turabian StyleMa, Tao, Feng Liang, Rongsheng Chen, Simin Liu, and Haijun Zhang. 2017. "Synthesis of Au-Pd Bimetallic Nanoflowers for Catalytic Reduction of 4-Nitrophenol" Nanomaterials 7, no. 9: 239. https://doi.org/10.3390/nano7090239
APA StyleMa, T., Liang, F., Chen, R., Liu, S., & Zhang, H. (2017). Synthesis of Au-Pd Bimetallic Nanoflowers for Catalytic Reduction of 4-Nitrophenol. Nanomaterials, 7(9), 239. https://doi.org/10.3390/nano7090239