Growth Method-Dependent and Defect Density-Oriented Structural, Optical, Conductive, and Physical Properties of Solution-Grown ZnO Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thin Film Seeds
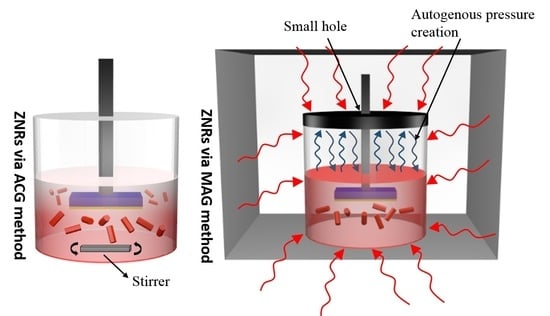
2.3. ZnO Nanorods Using ACG and MAG Methods
2.4. ZnO Nanoflowers Using ACG and MAG Methods
2.5. ZnO Nanotubes Using ACG and MAG Methods
2.6. Characterization Tools
3. Results and Discussion
3.1. Growth Mechanism and Internal Chemistry
3.2. Defect Density and Conductive Properties of ZNRs Grown with ACG and MAG Methods
3.3. Methodological Constraints for Nanorod Growth Via ACG and MAG Methods
3.4. Methodological Constraints for Nanoflower Growth via ACG and MAG Methods
3.5. Defect-Centric Etching of Nanorods into Nanotubes
3.6. XRD for ZNRs Grown with ACG and MAG Methods
3.7. ZNR Optical Properties from ACG and MAG Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patzke, G.R.; Zhou, Y.; Kontic, R.; Conrad, F. Oxide Nanomaterials: Synthetic Developments, Mechanistic Studies, and Technological Innovations. Angew. Chem. Int. Ed. 2011, 50, 826–859. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, D.M.; Chen, Y.F.; Zhu, Z.; Yao, T.; Koyama, S.; Shen, M.Y.; Goto, T. Optically Pumped Lasing of ZnO at Room Temperature. Appl. Phys. Lett. 1997, 70, 2230–2232. [Google Scholar] [CrossRef]
- Keis, K.; Vayssieres, L.; Rensmo, H.; Lindquist, S.E.; Hagfeldt, A. Photoelectrochemical Properties of Nano-to Microstructured ZnO Electrodes. J. Electrochem. Soc. 2001, 148, A149–A155. [Google Scholar] [CrossRef]
- Amin, M.; Manzoor, U.; Islam, M.; Bhatti, A.S.; Shah, N.A. Synthesis of ZnO nanostructures for low temperature CO and UV sensing. Sensors 2012, 12, 13842–13851. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Wang, Z.L. Spontaneous Polarization-Induced Nanohelixes, Nanosprings, and Nanorings of Piezoelectric Nanobelts. Nano Lett. 2003, 3, 1625–1631. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Donato, N.; Neri, G. CO and NO2 selective monitoring by ZnO-based sensors. Nanomaterials 2013, 3, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Konenkamp, R.; Word, R.C.; Schlegel, C. Vertical Nanowire Light-Emitting Diode. Appl. Phys. Lett. 2004, 85, 6004–6006. [Google Scholar] [CrossRef]
- Goldberger, J.; Sirbuly, D.J.; Law, M.; Yang, P. ZnO Nanowire Transistors. J. Phys. Chem. B 2005, 109, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-z Temperature Ultraviolet Nanowire Nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, J.; Nakagawa, N.; Hidaka, S.I.; Ueda, M.; Imasaka, K.; Higashihata, M.; Okada, T.; Hara, M. Dielectrophoretic Fabrication and Characterization of a ZnO Nanowire-Based UV Photosensor. Nanotechnology 2006, 17, 2567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chao, D.; Cui, H.; Zhang, W.; Zhang, W. Electronic structure and magnetism of Mn-Doped ZnO nanowires. Nanomaterials 2015, 5, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire Dye-Sensitized Solar Cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Al-Salman, H.S.; Abdullah, M.J. RF sputtering enhanced the morphology and photoluminescence of multi-oriented ZnO nanostructure produced by chemical vapor deposition. J. Alloys Compd. 2013, 547, 132–137. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled Growth of ZnO Nanowires and Their Optical Properties. Adv. Funct. Mater. 2002, 12, 323. [Google Scholar] [CrossRef]
- Park, W.I.; Yi, G.C.; Kim, M.; Pennycook, S.J. ZnO Nanoneedles Grown Vertically on Si Substrates by Non-Catalytic Vapor-Phase Epitaxy. Adv. Mater. 2002, 14, 1841–1843. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Ashfold, M.N. Growth of Aligned ZnO Nanorod Arrays by Catalyst-Free Pulsed Laser Deposition Methods. Chem. Phys. Lett. 2004, 396, 21–26. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Wang, N. Formation of ZnO Nanostructures by a Simple Way of Thermal Evaporation. Appl. Phys. Lett. 2002, 81, 757–759. [Google Scholar] [CrossRef]
- Rana, A.S.; Ko, K.; Hong, S.; Kang, M.; Kim, H.S. Fabrication and Characterization of ZnO Nanorods on Multiple Substrates. J. Nanosci. Nanotechnol. 2015, 15, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.S.; Kang, M.; Kim, H.S. Microwave-Assisted Facile and Ultrafast Growth of ZnO Nanostructures and Proposition of Alternative Microwave-Assisted Methods to Address Growth Stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Fu, M.; Zhao, A.; He, D.; Wang, Y. The effect of seed layer on morphology of ZnO nanorod arrays grown by hydrothermal method. J. Alloys Compd. 2010, 489, 99–102. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Fox, N.A.; Riley, D.J.; Ashfold, M.N. Synthesis of Aligned Arrays of Ultrathin ZnO Nanotubes on a Si Wafer Coated with a Thin ZnO Film. Adv. Mater. 2005, 17, 2477–2481. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Han, M.; Hao, X.; Zhu, F.A. General Low-Temperature Route for Large-Scale Fabrication of Highly Oriented ZnO Nanorod/Nanotube Arrays. J. Am. Chem. Soc. 2005, 127, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Lin, J.S.; Chen, Y.F. A simple approach for the growth of highly ordered ZnO nanotube arrays. J. Alloys Compd. 2009, 476, 903–907. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, L.; Chen, Z.; Jiang, Y.; Li, B.; Jia, Z.; Xu, L. Electrodeposition of ZnO Nanotube Arrays on TCO Glass Substrates. Electrochem. Commun. 2007, 9, 289–292. [Google Scholar] [CrossRef]
- Xu, L.; Liao, Q.; Zhang, J.; Ai, X.; Xu, D. Single-Rystalline ZnO Nanotube Arrays on Conductive Glass Substrates by Selective Dissolution of Electrodeposited ZnO Nanorods. J. Phys. Chem. C 2007, 111, 4549–4552. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Lindquist, S.E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Cheng, Q.; Ostrikov, K.K. Temperature-dependent growth mechanisms of low-dimensional ZnO nanostructures. CrystEngComm 2011, 13, 3455–3461. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, L.; Wang, Z.; Wang, Z.; Zhang, H.; Zhao, Y.; Liang, X. Epitaxial growth of ZnO thin films on Si substrates by PLD technique. J. Cryst. Growth 2005, 280, 455–461. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a Collapsing Bubble, Sonoluminescence and Conditions during Cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Flint, E.B.; Suslick, K.S. The temperature of cavitation. Science 1991, 253, 1397. [Google Scholar] [CrossRef] [PubMed]
- Jhung, S.H.; Jin, T.; Hwang, Y.K.; Chang, J.S. Microwave Effect in the Fast Synthesis of Microporous Materials: Which Stage between Nucleation and Crystal Growth is Accelerated by Microwave Irradiation? Chem. Eur. J. 2007, 13, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Lany, S.; Zunger, A. Anion Vacancies as a Source of Persistent Photoconductivity in II-VI and Chalcopyrite Semiconductors. Phys. Rev. B 2005, 72, 035215. [Google Scholar] [CrossRef]
- Look, D.C.; Hemsky, J.W.; Sizelove, J.R. Residual Native Shallow Donor in ZnO. Phys. Rev. Lett. 1999, 82, 2552. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Hydrogen Multicentre Bonds. Nat. Mater. 2007, 6, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.G.; Lander, J.J. Hydrogen as a Donor in Zinc Oxide. J. Chem. Phys. 1956, 25, 1136–1142. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, F. On the Origin of Intrinsic Donors in ZnO. Appl. Surf. Sci. 2010, 256, 3390–3393. [Google Scholar] [CrossRef]
- Zhang, Z.; Mu, J. Hydrothermal synthesis of ZnO nanobundles controlled by PEO–PPO–PEO block copolymers. J. Colloid Interface Sci. 2007, 307, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Israr, M.Q.; Sadaf, J.R.; Yang, L.L.; Nur, O.; Willander, M.; Palisaitis, J.; Persson, P.A. Trimming of Aqueous Chemically Grown ZnO Nanorods into ZnO Nanotubes and Their Comparative Optical Properties. Appl. Phys. Lett. 2009, 95, 073114. [Google Scholar] [CrossRef]
- McMurdie, H.F.; Morris, M.C.; Evans, E.H.; Paretzkin, B.; Wong-Ng, W.; Ettlinger, L.; Hubbard, C.R. Standard X-ray Diffraction Powder Patterns from the JCPDS Research Associateship. Powder Diffr. 1986, 1, 64–77. [Google Scholar] [CrossRef]
- Gupta, V.; Mansingh, A. Influence of Postdeposition Annealing on the Structural and Optical Properties of Sputtered Zinc Oxide Film. J. Appl. Phys. 1996, 80, 1063–1073. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wu, L.; Li, H.; Han, L.; Wang, B.; Xie, E. Effect of Annealing Atmosphere on the Photoluminescence of ZnO Nanospheres. Appl. Surf. Sci. 2009, 255, 4801–4805. [Google Scholar] [CrossRef]
- Keskenler, E.F.; Tomakin, M.; Dogan, S.; Turgut, G.; Aydın, S.; Duman, S.; Gurbulak, B. Growth and characterization of Ag/n-ZnO/p-Si/Al heterojunction diode by sol-gel spin technique. J. Alloys Compd. 2013, 550, 129–132. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, D.C.; Lee, J.Y.; Cho, H.K. Epitaxial Growth of ZnO Layers Using Nanorods with High Crystalline Quality. Nanotechnology 2007, 18, 395605. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.Y.; Chen, P.Y.; Wang, H.C.; Liao, C.H.; Chang, M.W.; Hsieh, Y.P.; Yang, C.C. Crystallinity Improvement of ZnO Thin Film on Different Buffer Layers Grown by MBE. J. Nanomater. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Alim, K.A.; Balandin, A.A.; Xiu, F.; Liu, J. Photoluminescence Investigation of the Carrier Recombination Processes in ZnO Quantum Dots and Nanocrystals. Phys. Rev. B 2006, 73, 165317. [Google Scholar] [CrossRef]
- Pal, U.; Serrano, J.G.; Santiago, P.; Xiong, G.; Ucer, K.B.; Williams, R.T. Synthesis and Optical Properties of ZnO Nanostructures with Different Morphologies. Opt. Mater. 2006, 29, 65–69. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms Behind Green Photoluminescence in ZnO Phosphor Powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Sardari, S.E.; Iliadis, A.A.; Stamataki, M.; Tsamakis, D.; Konofaos, N. Crystal quality and conductivity type of (002) ZnO films on (100) Si substrates for device applications. Solid State Electron. 2010, 54, 1150–1154. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B. Combined effects of screening and band gap renormalization on the energy of optical transitions in ZnO and GaN. J. Appl. Phys. 2000, 88, 5760–5763. [Google Scholar] [CrossRef]
- Fair, R.B. The effect of strain-induced band-gap narrowing on high concentration phosphorus diffusion in silicon. J. Appl. Phys. 1979, 50, 860–868. [Google Scholar] [CrossRef]







| Growth Method | Carrier Concentration (cm−3) | Resistivity (Ω∙cm) | Mobility (cm2/V-s) |
|---|---|---|---|
| ACG | 1.8 × 1017 | 31.8 | 1.05 |
| MAG | 1.08 × 1014 | 1010 | 56.8 |
| Growth Method | c (Å) | 2θ (Degree) | FWHM (Degree) | D (nm) | L (Å) | Strain (%) |
|---|---|---|---|---|---|---|
| ACG | 5.238 | 34.38 | 0.311 | 26.86 | 1.9751 | 63 |
| MAG | 5.201 | 34.43 | 0.122 | 68 | 1.9769 | 07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.U.H.S.; Lee, J.Y.; Shahid, A.; Kim, H.-S. Growth Method-Dependent and Defect Density-Oriented Structural, Optical, Conductive, and Physical Properties of Solution-Grown ZnO Nanostructures. Nanomaterials 2017, 7, 266. https://doi.org/10.3390/nano7090266
Rana AUHS, Lee JY, Shahid A, Kim H-S. Growth Method-Dependent and Defect Density-Oriented Structural, Optical, Conductive, and Physical Properties of Solution-Grown ZnO Nanostructures. Nanomaterials. 2017; 7(9):266. https://doi.org/10.3390/nano7090266
Chicago/Turabian StyleRana, Abu Ul Hassan Sarwar, Ji Young Lee, Areej Shahid, and Hyun-Seok Kim. 2017. "Growth Method-Dependent and Defect Density-Oriented Structural, Optical, Conductive, and Physical Properties of Solution-Grown ZnO Nanostructures" Nanomaterials 7, no. 9: 266. https://doi.org/10.3390/nano7090266
APA StyleRana, A. U. H. S., Lee, J. Y., Shahid, A., & Kim, H. -S. (2017). Growth Method-Dependent and Defect Density-Oriented Structural, Optical, Conductive, and Physical Properties of Solution-Grown ZnO Nanostructures. Nanomaterials, 7(9), 266. https://doi.org/10.3390/nano7090266