Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis
Abstract
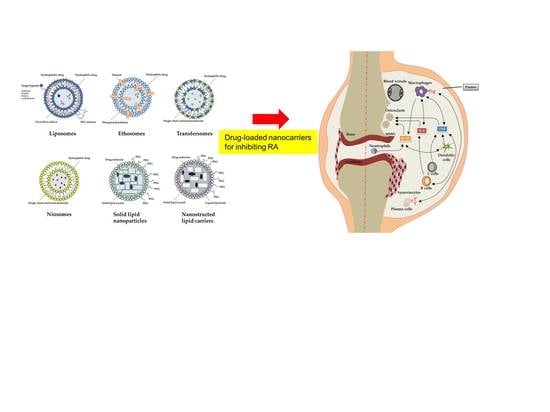
:1. Introduction
2. Anti-RA Activity of Lipid-Based Nanoparticles
3. Patents for RA Treatment by Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Semerano, L.; Minichiello, E.; Bessis, N.; Boissier, M.C. Novel Immunotherapeutic Avenues for Rheumatoid Arthritis. Trends Mol. Med. 2016, 22, 214–229. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Rantapaa-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, A.; Huizinga, T.W.; Worthington, J. The genetics of rheumatoid arthritis: Risk and protection in different stages of the evolution of RA. Rheumatology 2016, 55, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Esquide, V.; Sanmarti, R. Tobacco and other environmental risk factors in rheumatoid arthritis. Reumatol. Clin. 2012, 8, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Wu, D.; Trynka, G.; Raj, T.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Yoshida, S.; et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014, 506, 376–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, Y.; Terao, C.; Ikari, K.; Kochi, Y.; Ohmura, K.; Suzuki, A.; Kawaguchi, T.; Stahl, E.A.; Kurreeman, F.A.; Nishida, N.; et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat. Genet. 2012, 44, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Eyre, S.; Bowes, J.; Diogo, D.; Lee, A.; Barton, A.; Martin, P.; Zhernakova, A.; Stahl, E.; Viatte, S.; McAllister, K.; et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat. Genet. 2012, 44, 1336–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, E.A.; Raychaudhuri, S.; Remmers, E.F.; Xie, G.; Eyre, S.; Thomson, B.P.; Li, Y.; Kurreeman, F.A.; Zhernakova, A.; Hinks, A.; et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 2010, 42, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Boissier, M.C.; Semerano, L.; Challal, S.; Saidenberg-Kermanac’h, N.; Falgarone, G. Rheumatoid arthritis: From autoimmunity to synovitis and joint destruction. J. Autoimmun. 2012, 39, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kurreeman, F.A.; Padyukov, L.; Marques, R.B.; Schrodi, S.J.; Seddighzadeh, M.; Stoeken-Rijsbergen, G.; van der Helm-van Mil, A.H.; Allaart, C.F.; Verduyn, W.; Houwing-Duistermaat, J.; et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med. 2007, 4, e278. [Google Scholar]
- Remmers, E.F.; Plenge, R.M.; Lee, A.T.; Graham, R.R.; Hom, G.; Behrens, T.W.; de Bakker, P.I.; Le, J.M.; Lee, H.S.; Batliwalla, F.; et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 2007, 357, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.P.; Alfredsson, L.; Karlson, E.W. Environmental influences on risk for rheumatoid arthritis. Curr. Opin. Rheumatol. 2009, 21, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Vessey, M.P.; Villard-Mackintosh, L.; Yeates, D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception 1987, 35, 457–464. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. 3), S2. [Google Scholar] [CrossRef]
- Strehl, C.; van der Goes, M.C.; Bijlsma, J.W.; Jacobs, J.W.; Buttgereit, F. Glucocorticoid-targeted therapies for the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 2017, 26, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Rein, P.; Mueller, R.B. Treatment with Biologicals in Rheumatoid Arthritis: An Overview. Rheumatol. Ther. 2017, 4, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Juncker, T.; Schnekenburger, M.; Gaascht, F.; Diederich, M. Natural compounds as inflammation inhibitors. Genes Nutr. 2011, 6, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W.; O’Dell, J.R.; Paulus, H.E.; Curtis, J.R.; Bathon, J.M.; St Clair, E.W.; Bridges, S.L., Jr.; Zhang, J.; McVie, T.; Howard, G.; et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: The treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012, 64, 2824–2835. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Hossain, A.; Tanjong Ghogomu, E.; Mudano, A.S.; Maxwell, L.J.; Buchbinder, R.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E.; Tugwell, P.; Wells, G.A. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2017, 3, CD012591. [Google Scholar] [CrossRef] [PubMed]
- Soeken, K.L.; Miller, S.A.; Ernst, E. Herbal medicines for the treatment of rheumatoid arthritis: A systematic review. Rheumatology 2003, 42, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, W.; Lamprecht, A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. R. Soc. Interface 2010, 7 (Suppl. 1), S55–S66. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, D.; Colombo, M.; Zanoni, I.; Granucci, F. Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Semin. Immunol. 2017, 34, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Santamaria, P. Nanoparticle-based autoimmune disease therapy. Clin. Immunol. 2015, 160, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Singh, S.K.; Gulati, M.; Gupta, R.; Vaidya, Y. Application of liposomes in treatment of rheumatoid arthritis: Quo vadis. Sci. World J. 2014, 2014, 978351. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Feng, X.; Ding, J.; Chang, F.; Chen, X. Nanotherapeutics relieve rheumatoid arthritis. J. Control. Release 2017, 252, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Doshi, A.S.; Iyer, A.K.; Amiji, M.M. Multifunctional nanoparticles for targeting cancer and inflammatory diseases. J. Drug Target. 2013, 21, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, J.M.; van den Berg, W.B.; Holthuysen, A.E.; Wauben, M.H.; Storm, G.; van Lent, P.L. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Annals Rheum. Dis. 2004, 63, 348–353. [Google Scholar] [CrossRef]
- Hofkens, W.; Storm, G.; van den Berg, W.B.; van Lent, P.L. Liposomal targeting of glucocorticoids to the inflamed synovium inhibits cartilage matrix destruction during murine antigen-induced arthritis. Int. J. Pharm. 2011, 416, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Wardwell, P.R.; Forstner, M.B.; Bader, R.A. Investigation of the cytokine response to NF-κB decoy oligonucleotide coated polysaccharide based nanoparticles in rheumatoid arthritis in vitro models. Arthritis Res. Ther. 2015, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Zilberstein, A.C.; Jager, E.; Campos, M.M.; Morrone, F.B.; Calixto, J.B.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br. J. Pharmacol. 2009, 158, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Saini, M.K.; Thakur, K.; Kapil, N.; Garg, N.K.; Raza, K.; Goni, V.G.; Pareek, A.; Katare, O.P. Aceclofenac cocrystal nanoliposomes for rheumatoid arthritis with better dermatokinetic attributes: A preclinical study. Nanomedicine 2017, 12, 615–638. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Zhang, Y.; Crielaard, B.J.; Dusad, A.; Lele, S.M.; Rijcken, C.J.F.; Metselaar, J.M.; Kostkova, H.; Etrych, T.; Ulbrich, K.; et al. Nanomedicines for inflammatory arthritis: Head-to-head comparison of glucocorticoid-containing polymers, micelles, and liposomes. ACS Nano 2014, 8, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Rodgers, K.; Oliver, J.C.; Schluep, T. Alpha-methylprednisolone conjugated cyclodextrin polymer-based nanoparticles for rheumatoid arthritis therapy. Int. J. Nanomed. 2008, 3, 359–371. [Google Scholar]
- Ishihara, T.; Kubota, T.; Choi, T.; Higaki, M. Treatment of experimental arthritis with stealth-type polymeric nanoparticles encapsulating betamethasone phosphate. J. Pharmacol. Exp. Ther. 2009, 329, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Goodfellow, R.; Topley, N.; Amos, N.; Williams, B. The suppression of rat collagen-induced arthritis and inhibition of macrophage derived mediator release by liposomal methotrexate formulations. Inflamm. Res. 2000, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, W.; Na, K. Temperature-modulated noncovalent interaction controllable complex for the long-term delivery of etanercept to treat rheumatoid arthritis. J. Control. Release 2013, 171, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, D.; Sistla, R.; Ahmad, F.J.; Khar, R.K.; Diwan, P.V. Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug delivery. J. Biomed. Mater. Res. A 2007, 82, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Koning, G.A.; Schiffelers, R.M.; Wauben, M.H.; Kok, R.J.; Mastrobattista, E.; Molema, G.; ten Hagen, T.L.; Storm, G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006, 54, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Wang, H.; Jreyssaty, C.; Benderdour, M.; Lavigne, P.; Qiu, X.; Winnik, F.M.; Zhang, X.; Dai, K.; Shi, Q. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol. Ther. 2008, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, M.Y.; Bhang, S.H.; Kim, B.S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Heo, R.; Park, J.S.; Jang, H.J.; Kim, S.H.; Shin, J.M.; Suh, Y.D.; Jeong, J.H.; Jo, D.G.; Park, J.H. Hyaluronan nanoparticles bearing gamma-secretase inhibitor: In vivo therapeutic effects on rheumatoid arthritis. J. Control. Release 2014, 192, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Chan, H.W.; Wickline, S.A.; Lanza, G.M.; Pham, C.T. αVβ3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009, 23, 2978–2985. [Google Scholar] [CrossRef] [PubMed]
- Barrera, P.; Blom, A.; van Lent, P.L.; van Bloois, L.; Beijnen, J.H.; van Rooijen, N.; de Waal Malefijt, M.C.; van de Putte, L.B.; Storm, G.; van den Berg, W.B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1951–1959. [Google Scholar] [CrossRef]
- Chellat, F.; Merhi, Y.; Moreau, A.; Yahia, L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials 2005, 26, 7260–7275. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Bergstrom, K.; Osterberg, E.; Holmberg, K.; Hoffman, A.S.; Schuman, T.P.; Kozlowski, A.; Harris, J.H. Effects of branching and molecular weight of surface-bound poly(ethylene oxide) on protein rejection. J. Biomater. Sci. Polym. Ed. 1994, 6, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Tisdale, A.W.; Haidari, E.; Kokkoli, E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int. J. Pharm. 2009, 366, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Vehmeijer, L.J.; Kok, R.J.; Storm, G.; van Gaal, E.V. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Iwaszkiewicz, K.S.; Hua, S. Development of an effective topical liposomal formulation for localized analgesia and anti-inflammatory actions in the Complete Freund’s Adjuvant rodent model of acute inflammatory pain. Pain Phys. 2014, 17, E719–E735. [Google Scholar]
- Trif, M.; Guillen, C.; Vaughan, D.M.; Telfer, J.M.; Brewer, J.M.; Roseanu, A.; Brock, J.H. Liposomes as possible carriers for lactoferrin in the local treatment of inflammatory diseases. Exp. Biol. Med. 2001, 226, 559–564. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A controlled and novel drug delivery system. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Imam, S.S.; Aqil, M.; Amir, M.; Mir, S.R.; Mujeeb, M. Transdermal potential and anti-arthritic efficacy of ursolic acid from niosomal gel systems. Int. Immunopharmacol. 2015, 29, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sarwa, K.; Rudrapal, M.; Mazumder, B. Topical ethosomal capsaicin attenuates edema and nociception in arthritic rats. Drug Deliv. 2015, 22, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, X.; Zhou, Y.; Zhao, Y.; Ma, S.; Li, W.; Liu, Y.; Li, G. Enhanced topical delivery of tetrandrine by ethosomes for treatment of arthritis. Biomed Res. Int. 2013, 2013, 161943. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.P.; Cai, B.C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A novel vesicular carrier for enhanced transdermal delivery of sertraline: Development, characterization, and performance evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Preeti Kumar, M.S. Development of celecoxib transfersomal gel for the treatment of rheumatoid arthritis. Int. J. Pharm. Biol. Res. 2014, 2, 7–13. [Google Scholar]
- Sarwa, K.K.; Mazumder, B.; Rudrapal, M.; Verma, V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015, 22, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic Development of Transethosomal Gel System of Piroxicam: Formulation Optimization, In Vitro Evaluation, and Ex Vivo Assessment. AAPS PharmSciTech 2017, 18, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug Delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Madgulkar, A.R.; Desale, P.S.; Marium, G. Formulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritis. Drug Dev. Ind. Pharm. 2017, 43, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, Q.; Zhou, X.; Zhang, N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int. J. Pharm. 2008, 352, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, J.; Moura, C.C.; Sarmento, B.; Reis, S. Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics. Molecules 2015, 20, 11103–11118. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jiang, Z.Z.; Wu, T.; Li, J.; Zhang, L.; Zhao, Y.; Li, X.J.; Zhang, L.Y.; Yang, S.Y. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine 2012, 19, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Kuhad, A.; Kaur, I.P.; Chopra, K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain 2015, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Tyagi, R.K.; Sharma, G.; Katare, O.P. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surf. B Biointerfaces 2016, 147, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kawadkar, J.; Pathak, A.; Kishore, R.; Chauhan, M.K. Formulation, characterization and in vitro-in vivo evaluation of flurbiprofen-loaded nanostructured lipid carriers for transdermal delivery. Drug Dev. Ind. Pharm. 2013, 39, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.B.; Tavares, E.R.; Guido, M.C.; Bonfa, E.; Maranhao, R.C. Anti-inflammatory effects of intravenous methotrexate associated with lipid nanoemulsions on antigen-induced arthritis. Clinics 2016, 71, 54–58. [Google Scholar] [CrossRef]
- Pozzi, F.S.; Maranhao, R.C.; Guedes, L.K.; Borba, E.F.; Laurindo, I.M.; Bonfa, E.; Vinagre, C.G. Plasma kinetics of an LDL-like non-protein nanoemulsion and transfer of lipids to high-density lipoprotein (HDL) in patients with rheumatoid arthritis. J. Clin. Lipidol. 2015, 9, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B. A review of sarilumab for the treatment of rheumatoid arthritis. Immunotherapy 2018, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Dahal, K.; Sharma, S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2016, 36, 1065–1075. [Google Scholar] [CrossRef] [PubMed]


| Therapeutic Classification | Therapeutic Category | Drugs/Agents | Mechanism of Action | Side Effect | Reference |
|---|---|---|---|---|---|
| NSAIDs | - | Aspirin, celecoxib, indometacin, ibuprofen | COXs inhibitors, Immunomodulation | Gastrointestinal reaction, dysfunction of kidney, etc. | [16] |
| Glucocorticoids | - | Dexamethasone, hydrocortisone, prednisone and methylprednisolone | Immunosuppression | Hyperadrenocorticism, infection, hypertension and atherosclerosis, osteoporosis and osteonecrosis, etc. | [17] |
| DMARDs | - | Methotrexate, hydroxychloroquine, sulfasalazine, clodronate and leflunomide | Immunosuppression, Disease-modifying activity | Myelosuppression, gastrointestinal reaction, dysfunction of liver and kidney, etc. | [18] |
| Biological agents | Anti-cytokines | Anakinra, Sarilumab, tocilizumab | IL-1 receptor | Infection | [19] |
| Sarilumab, tocilizumab | Interlukin-6R inhibitor | Infection, gastrointestinal perforation | |||
| Sirukumab, olokizumab, siltuximab | Interlukin-6 inhibitor | Infection, gastrointestinal perforation | |||
| Etanercept, adalimumab, ifliximab, certolizumab pegol, golimumab | TNF-α inhibitor | Infection, tuberculosis | |||
| Anti-T cell | Abatacept | Co-stimulation inhibitors | Infection, malignancy | ||
| Anti-B cell | Rituximab | B-cell depletion (anti-CD20) | Infection, hypertension | ||
| Kinase inhibitors | Baricitinib, tofacitinib | Janus kinase(JAK)1 and 2 inhibitor | Infection | ||
| Natural products | - | Curcumin, Resveratrol, Guggulsterone, Withanolide | IL-6, COX-2, TNF-α | - | [20] |
| Therapeutic Classification | Drugs/Agents | Nanocarrier System | Mean Size (nm) | Delivery/Target | Model | Reference |
|---|---|---|---|---|---|---|
| NSAIDs | Indomethacin | Polymeric micelles | 240 | EPR | AIA | [37] |
| Aceclofenac | Lysine-liposomes | - | EPR | AIA | [38] | |
| Indomethacin | Folate-PEG-PAMAM dendrimer | <100 | Folate receptor (macrophages) | Patients | [45] | |
| Indomethacin | Lipid microspheres | 150 | EPR | AIA | [54] | |
| Glucocorticoids | Dexamethasone | Liposomes | 96 | EPR | AIA | [39] |
| Methylprednisolone | Cyclodextrin polymer | 27 | EPR | CIA | [40] | |
| Dexamethasone | RGD-PEG liposomes | 100 | Endothelials | AIA | [46] | |
| DMARDs | Methotrexate | Stealth-type polymeric nanoparticles | 51–116 | EPR | AIA | [41] |
| Methotrexate | PEGylated liposomes | 210–260 | EPR | AIA | [42] | |
| Clodronate | Liposomes | 120–160 | Macrophages | AIA | [52] | |
| Biological agents | Etanercept | TMN complex | 250 | EPR | CIA | [43] |
| Anakinra | Folate-chitosan DNA nanoparticles | 110 | Macrophages | AIA | [47] | |
| Tocilizumab | Hyaluronate-gold nanoparticles | 64 | IL-6R+ cells | CIA | [48] | |
| Others inhibitor | γ-secretase inhibitor | Hyaluronan nanoparticles | 255 | Macrophages | CIA | [50] |
| Fumagillin | Perfluorocarbon nanoparticle | 250 | αVβ3 integrin activated cells | K/BxN mouse model | [51] |
| Lipid Nanocarrier | Drugs | Mean Size (nm) | Route of Administration | In Vitro/In Vivo Studies | Reference |
|---|---|---|---|---|---|
| Liposomes | Loperamide | 102 | Topical | AIA | [54] |
| Lactoferrin | - | SC | AIA | [63] | |
| Niosomes | Ursolic acid | 665 | Topical | AIA | [65] |
| Luteolin | 534–810 | Topical | AIA | [66] | |
| Ethosomes | Capsaicin | 217–295 | Topical | Rat skin | [68] |
| Tetrandrine | 78 | Topical | Rat skin | [69] | |
| Transfersomes | Capsaicin | 94 | Topical | AIA | [73] |
| Celecoxib | 100 | Topical | Rat skin | [72] | |
| Piroxicam | 655 | Topical | Porcine skin | [74] | |
| SLN | Piperine | 128 | Oral and topical | AIA | [79] |
| Actarit | 241 | IV | Patients | [80] | |
| Methotrexate | 250 | - | THP-1 cells | [81] | |
| Tripterygium | 116 | Oral | AIA | [82] | |
| Curcumin | 134 | Oral | AIA | [83] | |
| NLC | Methotrexate | 181 | Topical | AIA | [87] |
| Flurbiprofen | 214 | Topical | Carrageenan-induced rat paw edema | [88] | |
| lipid nanoemusion | Methotrexate | - | IV | AIA | [89] |
| Low-density lipoprotein and high-density lipoprotein | 148 | IV | Patients | [90] |
| Patent | Lipid Nanocarrier | Advantage Function |
|---|---|---|
| US 20150174069 A1 | Dexamethasone sodium phosphate liposome | There is about a 10% reduction in one or more symptoms of arthritis |
| WO 2003000190 A2 | Glycosaminoglycans liposome | It provides good efficacy in treatment of osteoarthritis |
| CN 104688721 A | Paclitaxel liposome | The gel achieves a treatment effect and pain of a patient suffering from RA |
| US 20090232731 A1 | Cationic liposome | It provides reduction of the infiltration of mononuclear cells into the synovial tissue, pannus development and cartilage erosion |
| US 20160000714 | Curcumin solid lipid particles | It provides suppression of cyclooxygenase 2 (COX-2) expression |
| WO 2017025588 A1 | Cyclosporine solid lipid particles | It prevents transcription of interleukin 2, thereby decreasing activation and proliferation of T lymphocytes. |
| US 8715736 B2 | Nanostructured Lipid Carriers | It provides efficient skin permeation at the inflammatory site in RA |
| CN 102225205 B | Tripterine nanostructured lipid carrier | It provides inhibition of rheumatoid arthritis inflammation |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, S.-Y.; Lin, C.-H.; Huang, T.-H.; Fang, J.-Y. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials 2018, 8, 42. https://doi.org/10.3390/nano8010042
Chuang S-Y, Lin C-H, Huang T-H, Fang J-Y. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials. 2018; 8(1):42. https://doi.org/10.3390/nano8010042
Chicago/Turabian StyleChuang, Shih-Yi, Chih-Hung Lin, Tse-Hung Huang, and Jia-You Fang. 2018. "Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis" Nanomaterials 8, no. 1: 42. https://doi.org/10.3390/nano8010042
APA StyleChuang, S. -Y., Lin, C. -H., Huang, T. -H., & Fang, J. -Y. (2018). Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials, 8(1), 42. https://doi.org/10.3390/nano8010042