Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nano-Fe3O4/Fiber Composite
2.3. Characterization
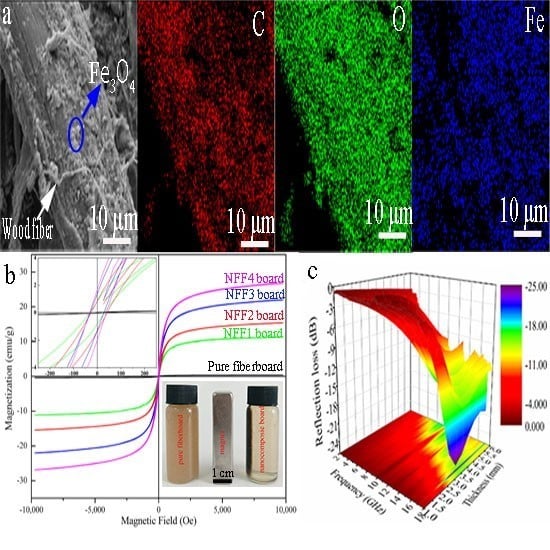
2.3.1. Composite Structure
2.3.2. Electromagnetic Test
2.3.3. Mechanical Studies
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, D.D.L. Carbon materials for structural self-sensing, electromagnetic shielding and thermal interfacing. Carbon 2012, 50, 3342–3353. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhang, H.-B.; Li, X.; Gui, C.-X.; Yu, Z.-Z. Enhanced electromagnetic interference shielding efficiency of polystyrene/graphene composites with magnetic Fe3O4 nanoparticles. Carbon 2015, 82, 67–76. [Google Scholar] [CrossRef]
- Ding, Z.; Shi, S.Q.; Zhang, H.; Cai, L. Electromagnetic shielding properties of iron oxide impregnated kenaf bast fiberboard. Compos. Part B 2015, 78, 266–271. [Google Scholar] [CrossRef]
- Kong, I.; Hj Ahmad, S.; Hj Abdullah, M.; Hui, D.; Nazlim Yusoff, A.; Puryanti, D. Magnetic and microwave absorbing properties of magnetite–thermoplastic natural rubber nanocomposites. J. Magn. Magn. Mater. 2010, 322, 3401–3409. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Li, G.; Tang, J.; Wang, T.; Guo, Y.; Xue, H. Laminated magnetic graphene with enhanced electromagnetic wave absorption properties. J. Mater. Chem. C 2013, 1, 765–777. [Google Scholar] [CrossRef]
- Maksymov, I.S. Magneto-plasmonics and resonant interaction of light with dynamic magnetisation in metallic and all-magneto-dielectric nanostructures. Nanomaterials 2015, 5, 577–613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Wei, S.; Zhang, B.; Yu, M.; Zhao, W.; Liu, J. Fabrication of porous graphene-Fe3O4 hybrid composites with outstanding microwave absorption performance. Compos. Part A 2017, 95, 237–247. [Google Scholar] [CrossRef]
- Kong, L.B.; Li, Z.W.; Liu, L.; Huang, R.; Abshinova, M.; Yang, Z.H.; Tang, C.B.; Tan, P.K.; Deng, C.R.; Matitsine, S. Recent progress in some composite materials and structures for specific electromagnetic applications. Int. Mater. Rev. 2013, 58, 203–259. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Löbmann, K.; Wohlert, J.; Müllertz, A.; Wågberg, L.; Svagan, A.J. Cellulose nanopaper and nanofoam for patient-tailored drug delivery. Adv. Mater. Interfaces 2017, 4, 1600655. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Huber, T.; Pang, S.; Staiger, M.P. All-cellulose composite laminates. Compos. Part A 2012, 43, 1738–1745. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Paillet, M.; Dufresne, A. Tangling Effect in Fibrillated Cellulose Reinforced Nanocomposites. Macromolecules 2004, 37, 4313–4316. [Google Scholar] [CrossRef]
- Sapkota, J.; Shirole, A.; Foster, E.J.; Garcia, J.C.M.; Lattuada, M.; Weder, C. Polymer nanocomposites with nanorods having different length distributions. Polymer 2016, 110. [Google Scholar] [CrossRef]
- Altaner, C.; Thomas, L.H.; Fernandes, A.N.; Jarvis, M.C. How Cellulose Stretches: Synergism between Covalentand Hydrogen Bonding. Biomacromolecules 2014, 15, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crop. Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Qing, Y.; Wang, X.; Zhou, Y.; Huang, Z.; Luo, F.; Zhou, W. Enhanced microwave absorption of multi-walled carbon nanotubes/epoxy composites incorporated with ceramic particles. Compos. Sci. Technol. 2014, 102, 161–168. [Google Scholar] [CrossRef]
- Dang, B.; Chen, Y.; Shen, X.; Chen, B.; Sun, Q.; Jin, C. Fabrication of a nano-ZnO/polyethylene/wood-fiber composite with enhanced microwave absorption and photocatalytic activity via a facile hot-press method. Materials 2017, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Kara, A.; Schröder, E.; Hyldgaard, P.; Rahman, T.S. Physisorption of nucleobases on graphene: A comparative van der Waals study. J. Phys. Condens. Matter. 2012, 24, 424210. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Hao, C.; Ye, J.; Yu, R.; Huang, D. Graphene-enhanced microwave absorption properties of Fe3O4/SiO2 nanorods. Compos. Part A 2016, 89, 40–46. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Z.; Wan, G.; Lin, S.; Yang, P.; Qin, Y. High densities of magnetic nanoparticles supported on graphene fabricated by atomic layer deposition and their use as efficient synergistic microwave absorbers. Nano Res. 2014, 7, 704–716. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly mesoporous organic aerogels derived from soy and tannin. Green Chem. 2012, 14, 3099–3106. [Google Scholar] [CrossRef]
- Sheng, C.; Wang, C.; Wang, H.; Jin, C.; Sun, Q.; Li, S. Self-photodegradation of formaldehyde under visible-light by solid wood modified via nanostructured Fe-doped WO3 accompanied with superior dimensional stability. J. Hazard. Mater. 2017, 328, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Yao, Q.; Fan, B.; Wang, C.; Xiong, Y.; Jin, C.; Sun, Q. Biomimetic taro leaf-like films decorated on wood surfaces using soft lithography for superparamagnetic and superhydrophobic performance. J. Mater. Sci. 2017, 52, 7428–7438. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Z.; Guo, H.; Li, X.; Wang, J.; Huang, S.; Gan, L. Fe2O3 particles enwrapped by graphene with excellent cyclability and rate capability as anode materials for lithium ion batteries. Appl. Surf. Sci. 2013, 266, 148–154. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Dang, B.; Xiong, Y.; Yao, Q.; Wang, C.; Sun, Q.; Jin, C. Bio-Inspired nacre-like nanolignocellulose-poly (vinyl alcohol)-TiO2 composite with superior mechanical and photocatalytic properties. Sci. Rep. 2017, 7, 1823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Gao, H.; Huang, Y.; Liu, X.; Li, J.; Xu, X.; Wang, X.K. Formation of Fe3O4@MnO2 ball-in-ball hollow spheres as a high performance catalyst for enhanced catalytic performances. J. Mater. Chem. A 2016, 4, 1414–1422. [Google Scholar] [CrossRef]
- Ren, P.-G.; Wang, H.; Yan, D.-X.; Huang, H.-D.; Wang, H.-B.; Zhang, Z.-P.; Xu, L.; Li, Z.-M. Ultrahigh gas barrier poly (vinyl alcohol) nanocomposite film filled with congregated and oriented Fe3O4@GO sheets induced by magnetic-field. Compos. Part A 2017, 97, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Q.; Wang, C.; Ma, Z.; Sun, Q.; Fan, B.; Jin, C.; Chen, Y. Hydrothermal synthesis of nanooctahedra MnFe2O4 onto the wood surface with soft magnetism, fire resistance and electromagnetic wave absorption. Nanomaterials 2017, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.D.; Li, J.; Zheng, R.X. Thermal and combustion characteristics of binderless fiberboard. Adv. Mater. Res. 2010, 113, 1063–1070. [Google Scholar] [CrossRef]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631–1640. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, P.; She, X.; Xia, Y.; Komarneni, S.; Xi, K.; Che, Y.; Yao, X.; Yang, D. Sustainable seaweed-based one-dimensional (1D) nanofibers as high-performance electrocatalysts for fuel cells. J. Mater. Chem. A 2015, 3, 14188–14194. [Google Scholar] [CrossRef]
- Cui, J.; Xi, Y.; Chen, S.; Li, D.; She, X.; Sun, J.; Han, W.; Yang, D.; Guo, S. Prolifera-Green-Tide as Sustainable Source for Carbonaceous Aerogels with Hierarchical Pore to Achieve Multiple Energy Storage. Adv. Funct. Mater. 2016, 26, 8487–8495. [Google Scholar] [CrossRef]
- He, Q.; Yuan, T.; Wei, S.; Haldolaarachchige, N.; Luo, Z.; Young, D.P.; Khasanov, A.; Guo, Z. Morphology- and Phase-Controlled Iron Oxide Nanoparticles Stabilized with Maleic Anhydride Grafted Polypropylene. Angew. Chem. Int. Edit. 2012, 51, 8842–8845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ren, J.; Yang, X.; Chang, G.; Bu, Y.; Wei, G.; Han, W.; Yang, D. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 9952. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.; Lin, X.; Shen, X.; Jia, J.; Sharif, F.; Kim, J.K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Jucius, D.; Kopustinskas, V.; Grigaliūnas, V.; Guobienė, A.; Lazauskas, A.; Andrulevičius, M. Highly hydrophilic poly(ethylene terephthalate) films prepared by combined hot embossing and plasma treatment techniques. Appl. Surface Sci. 2015, 349, 200–210. [Google Scholar] [CrossRef]
- Fu, K.; Huang, J.; Yao, N.; Xu, X.; Wei, M. Enhanced Photocatalytic Activity Based on Composite Structure with Downconversion Material and Graphene. Ind. Eng. Chem. Res. 2016, 55, 1559–1565. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A 2012, 1, 959–965. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, S.; Yang, X.; Ma, N.; Xia, Y.; Yang, D.; Guo, S. Suppressing Fe–Li Antisite Defects in LiFePO4/Carbon Hybrid Microtube to Enhance the Lithium Ion Storage. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse Magnetic Single-Crystal Ferrite Microspheres. Angew. Chem. Int. Edit. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Nairan, A.; Khan, U.; Iqbal, M.; Khan, M.; Javed, K.; Riaz, S.; Naseem, S.; Han, X. Structural and magnetic response in bimetallic core/shell magnetic nanoparticles. Nanomaterials 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Govindan, B.; Latha, B.S.; Nagamony, P.; Ahmed, F.; Saifi, M.A.; Harrath, A.H.; Alwasel, S.; Mansour, L.; Alsharaeh, E.H. Designed synthesis of nanostructured magnetic hydroxyapatite based drug nanocarrier for anti-cancer drug delivery toward the treatment of human epidermoid carcinoma. Nanomaterials 2017, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shao, G.; Fan, B.; Zhao, W.; Xie, Y.; Zhang, R. Facile preparation and enhanced microwave absorption properties of core-shell composite spheres composited of Ni cores and TiO2 shells. Phys. Chem. Chem. Phys. 2015, 17, 8802–8810. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Bao, C.; Qian, X.; Song, L.; Tai, Q.; Liew, K.M.; Hu, Y. Design of artificial nacre-like hybrid films as shielding to mitigate electromagnetic pollution. Carbon 2014, 75, 178–189. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Widyorini, R.; Xu, J.; Umemura, K.; Kawai, S. Manufacture and properties of binderless particleboard from bagasse I: Effects of raw material type, storage methods, and manufacturing process. J. Wood Sci. 2005, 51, 648. [Google Scholar] [CrossRef]
- Cofrades, S.; Guerra, M.; Carballo, J.; Fernández-Martín, F.; Colmenero, F.J. Plasma protein and soy fiber content effect on bologna sausage properties as influenced by fat level. J. Food Sci. 2000, 65, 281–287. [Google Scholar] [CrossRef]
- Álvarez, C.; Rojano, B.; Almaza, O.; Rojas, O.J.; Gañán, P. Self-Bonding Boards From Plantain Fiber Bundles After Enzymatic Treatment: Adhesion Improvement of Lignocellulosic Products by Enzymatic Pre-Treatment. J. Polym. Environ. 2011, 19, 182–188. [Google Scholar] [CrossRef]
- Sitz, E.D.; Bajwa, D.S. The mechanical properties of soybean straw and wheat straw blended medium density fiberboards made with methylene diphenyl diisocyanate binder. Ind. Crop. Prod. 2015, 75, 200–205. [Google Scholar] [CrossRef]













| Sample | C (at %) | O (at %) | Fe (at %) | C/O Ratio |
|---|---|---|---|---|
| Fiber | 68.63 | 31.37 | - | 2.40 |
| NFF1 | 67.33 | 31.75 | 0.92 | 2.12 |
| NFF2 | 63.64 | 33.82 | 2.54 | 1.88 |
| NFF3 | 61.59 | 33.58 | 4.83 | 1.83 |
| NFF4 | 56.55 | 37.67 | 5.78 | 1.50 |
| Sample | C (at %) | O (at %) | Fe (at %) | C/O ratio |
|---|---|---|---|---|
| Fiberboard | 68.48 | 31.52 | - | 2.17 |
| NFF1 board | 64.53 | 34.5 | 0.97 | 1.87 |
| NFF2 board | 60.55 | 37.5 | 1.95 | 1.61 |
| NFF3 board | 57.87 | 39.68 | 2.45 | 1.46 |
| NFF4 board | 55.93 | 40.39 | 3.68 | 1.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, B.; Chen, Y.; Wang, H.; Chen, B.; Jin, C.; Sun, Q. Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method. Nanomaterials 2018, 8, 52. https://doi.org/10.3390/nano8010052
Dang B, Chen Y, Wang H, Chen B, Jin C, Sun Q. Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method. Nanomaterials. 2018; 8(1):52. https://doi.org/10.3390/nano8010052
Chicago/Turabian StyleDang, Baokang, Yipeng Chen, Hanwei Wang, Bo Chen, Chunde Jin, and Qingfeng Sun. 2018. "Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method" Nanomaterials 8, no. 1: 52. https://doi.org/10.3390/nano8010052
APA StyleDang, B., Chen, Y., Wang, H., Chen, B., Jin, C., & Sun, Q. (2018). Preparation of High Mechanical Performance Nano-Fe3O4/Wood Fiber Binderless Composite Boards for Electromagnetic Absorption via a Facile and Green Method. Nanomaterials, 8(1), 52. https://doi.org/10.3390/nano8010052