Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
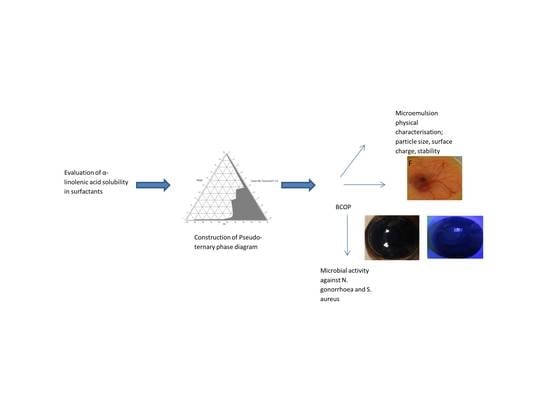
2.1. Saturation Solubility
2.2. Construction of Pseudo-Ternary Phase Diagrams
2.3. Preparation and Characterization of Microemulsions
2.3.1. Characterization of Microemulsions
2.3.2. Contact Angle Measurements
2.3.3. Self-Diffusion NMR
2.3.4. Bovine Corneal Opacity and Permeability (BCOP) Test
2.3.5. Hen’s Egg Test Chorioallantoic Membrane (HET-CAM)
2.3.6. Antibacterial Activity of Microemulsions against N. gonorrhoeae and S. aureus
3. Materials and Methods
3.1. Materials
3.2. Microorganisms
3.3. Preformulation Studies
3.3.1. Excipient Selection (Surfactants & Co-Surfactants)
3.3.2. Determination of Saturation Solubility of α-Linolenic Acid in Different Surfactants and Co-Surfactants
3.3.3. Selection of Surfactant & Co-Surfactant Blend
3.3.4. Construction of Pseudo-Ternary Phase Diagram
3.3.5. Preparation of Microemulsion Formulations
3.3.6. Characterization of Microemulsions
Visual Evaluation
Polarized Light Microscopy
Droplet Size and Zeta Potential Measurement
Determination of pH
Viscosity Measurements
Drug Content Determination
Contact Angle Measurements
3.3.7. Stability Studies
3.3.8. Self-Diffusion NMR
3.3.9. Antibacterial Activity of MEs against N. gonorrhoea and S. aureus
3.3.10. Ocular Irritation Testing
BCOP Test
HET-CAM Test
3.3.11. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Di Bartolomeo, S.; Mirta, D.H.; Janer, M.; Rodriguez, M.R.; Sauka, D.; Magarinos, F.; De Torres, R.A. Incidence of Chlamydia trachomatis and other potential pathogens in neonatal conjunctivitis. Int. J. Infect. 2001, 5, 139–143. [Google Scholar] [CrossRef]
- Kellogg, D.S., Jr.; Peacock, W.L., Jr.; Deacon, W.E.; Brown, L.; Pirkle, D.I. Neisseria Gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. J. Bacteriol. 1963, 85, 1274–1279. [Google Scholar] [PubMed]
- Matejcek, A.; Goldman, R.D. Treatment and prevention of ophthalmia neonatorum. Can. Fam. Physician 2013, 59, 1187–1190. [Google Scholar] [PubMed]
- Palafox, S.K.V.; Jasper, S.; Allyson, D.; Foster, S.C. Ophthalmia Neonatorum. J. Clin. Exp. Ophthalmol. 2011, 2, 119. [Google Scholar] [CrossRef]
- Laga, M.; Meheus, A.; Piot, P. Epidemiology and control of Gonococcal ophthalmia neonatorum. Bull. World Health Organ. 1989, 67, 471–477. [Google Scholar] [PubMed]
- Laga, M.; Plummer, F.A.; Piot, P.; Datta, P.; Namaara, W.; Ndinya-Achola, J.O.; Nzanze, H.; Maitha, G.; Ronald, R.A.; Pamba, O.H. Prophylaxis of Gonococcal and chlamydial ophthalmia neonatorum. A comparison of silver nitrate and tetracycline. N. Engl. J. Med. 1988, 318, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Resenberg, H.M. Silver nitrate ophthalmic solution and chemical conjunctivitis. Pediatrics 1975, 56, 368–373. [Google Scholar] [PubMed]
- Bergsson, G.; Steingrimsson, O.; Thormar, H. In vitro susceptibilities of Neisseria Gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1999, 43, 2790–2792. [Google Scholar] [PubMed]
- Lepage, P.; Bogaerts, J.; Kestelyn, P.; Meheus, A. Single-dose cefotaxime intramuscularly cures gonococcal ophthalmia neonatorum. Br. J. Ophthalmol. 1988, 72, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.; Watanabe, H.; Kitawaki, J. Ceftriaxone-resistant Neisseria Gonorrhoeae, Japan. Emerg. Infect. Dis. 2011, 17, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.; Kitawaki, J.; Unemo, M. Is Neisseria Gonorrhoeae initiating a future era of untreatable Gonorrhoea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Miller, R.D.; Brown, K.E.; Morse, S.A. Inhibitory action of Fatty acids on the growth of Neisseria Gonorrhoeae. Infect. Immun. 1977, 17, 303–312. [Google Scholar] [PubMed]
- Desbois, A.P.; Lawlor, K.C. Antibacterial Activity of Long-Chain Polyunsaturated Fatty Acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Bergsson, G.; Gunnarsson, E.; Georgsson, G.; Witvrouw, M.; Steingrimsson, O.; De Clercq, E.; Kristmundsdottir, T. Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex. Transm. Infect. 1999, 75, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Butt, U.; ElShaer, A.; Snyder, L.A.; Chaidemenou, A.; Alany, R.G. Fatty acid microemulsion for the treatment of neonatal conjunctivitis: Quantification, characterisation and evaluation of antimicrobial activity. Drug Deliv. Transl. Res. 2016, 6, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.; Churchward, C.; Alany, R.; Kirk, R.S.; Walker, T. Prevention of ophthalmia neonatorum from ‘Neisseria gonorrhoea’ using a fatty acid-based formulation. mBio 2017, 8. [Google Scholar] [CrossRef]
- Keenan, J.; Eckert, S.; Rutar, T. Cost analysis of povidone-iodine for ophthalmia neonatorum prophylaxis. Arch. Ophthalmol. 2010, 128, 136–137. [Google Scholar] [PubMed]
- Patel, K.; Sarma, V.; Vavia, P. Design and evaluation of Lumefantrine—Oleic acid self-nanoemulsifying ionic complex for enhanced dissolution. DARU J. Pharm. Sci. 2013, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Cuomo, F.; Lopez, F.; Ceglie, A. Role of emulsifier layer, antioxidants and radical initiators in the oxidation of olive oil-in-water emulsions. Food Res. Int. 2013, 50, 377–383. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Boonme, P.; Wurster, D.E.; Rades, T. Aerosol OT microemulsions as carriers for transdermal delivery of hydrophobic and hydrophilic local anesthetics. Drug Deliv. 2008, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M.; Jockovic, J. Phase behaviour, microstructure and ibuprofen solubilization capacity of pseudo-ternary non-ionic microemulsions. J. Mol. Liq. 2011, 160, 81–87. [Google Scholar] [CrossRef]
- Tarr, B.D.; Yalkowsky, S.H. Enhanced intestinal absorption of cyclosporin in rats through the reduction of emulsion droplet size. Pharm. Res. 1989, 6, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pang, X.; Zhang, W.; Wang, S. Silymarin-loaded self microemulsifying drug delivery systems. Asian J. Pharm. Sci. 2007, 2, 150–160. [Google Scholar]
- James-Smith, M.A.; Alford, K.; Shah, D.O. A novel method to quantify the amount of surfactant at the oil/water interface and to determine total interfacial area of emulsions. J. Colloid Interface Sci. 2007, 310, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Chhabra, V.; Pagidipati, R.; Shah, R.; Shah, D.O. Effect of chain length compatibility on micellar stability in sodium dodecyl sulfate/alkyltrimethylammonium bromide solutions. Langmuir 1997, 13, 432–434. [Google Scholar] [CrossRef]
- Adhikary, T.P.; Chowdhury, P.; Chakravorti, S. Modulation of photophysics of 2-hydroxy-1-naphthaldehyde in non-ionic micelles. Chem. Phys. Lett. 2007, 442, 504–510. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Parikh, J.R.; Solanki, A.B.; Patel, B.G. Effect of formulation components on the in vitro permeation of microemulsion drug delivery system of fluconazole. AAPS Pharm. Sci. Tech. 2009, 10, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.S.; Yadav, E.; Verma, A.; Amin, S. Development, Characterization and Pharmacodynamic Evaluation of Hydrochlorothiazide Loaded Self-Nanoemulsifying Drug Delivery Systems. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Niculae, G.; Lǎcǎtuşu, I.; Badea, N.; Opera, O.; Meghea, A. Optimization of lipid nanoparticles composition for sunscreen encapsulation. U.P.B. Sci. Bull. Ser. B. 2013, 75, 79–92. [Google Scholar]
- Gupta, S.; Bansal, B.; Ali, J.; Gabrani, R.; Dang, S. Development and Characterization of Polyphenon 60 and Caffeine Microemulsion for Enhanced Antibacterial Activity. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Allen, R.R.; Jackson, A.; Kummerow, F.A. Factors which affect the stability of highly unsaturated fatty acids. I. Differences in the oxidation of conjugated and nonconjugated linoleic acid. J. Am. Oil Chem. Soc. 1949, 26, 395–399. [Google Scholar] [CrossRef]
- Wang, W.; Wei, H.; Du, Z.; Tai, X.; Wang, G. Formation and Characterization of fully dilutable microemulsion with fatty acid methyl esters as oil phase. ACS Sustain. Chem. Eng. 2015, 3, 443–450. [Google Scholar] [CrossRef]
- Montgomery, T.M. Anatomy, Physiology & Pathology of the Human Eye: The Cornea. Available online: http://www.tedmontgomery.com/the_eye/cornea.html (accessed on 25 July 2017).
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties: Surface Science Techniques; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 1998; Volume 51, pp. 3–34. [Google Scholar]
- Kwok, D.Y.; Neumann, A.W. Contact angle techniques and measurements. In Surface Characterization Methods: Principles, Techniques and Applications; Milling, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1999; p. 37. [Google Scholar]
- Boonme, P.; Krauel, K.; Graf, A.; Rades, T.; Junyaprasert, V.B. Characterisation of microstructures formed in isopropyl palmitate/water/Aerosol OT: 1-butanol (2:1) system. Pharmazie 2006, 61, 927–932. [Google Scholar] [PubMed]
- Boonme, P.; Krauel, K.; Graf, A.; Rades, T.; Junyaprasert, V.B. Characterization of Microemulsion Structures in the Pseudoternary Phase Diagram of Isopropyl Palmitate/Water/Brij 97:1 Butanol. AAPS Pharm. Sci. Tech. 2006, 7, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Lang, Y.; Yao, H.; Dong, Y.; Li, S. Bicontinuous cyclosporin a loaded water-AOT/Tween 85-isopropylmyristate microemulsion: Structural characterization and dermal pharmacokinetics in vivo. J. Pharm. Sci. 2009, 98, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Woodman, T.J. Applications of NMR in the characterization of pharmaceutical microemulsions. J. Control Release 2012, 161, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Ismail, S.; Hussein, A.; Wua, Z.; Al-Kassasa, R.; Alany, R.G. Conjunctival and corneal tolerability assessment of ocular naltrexone noisome and their ingredients on the hen’s egg chorioallantoic membrane and excised bovine cornea models. Int. J. Pharm. 2012, 432, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y.S. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores. Open Access 2016, 5, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Nie, S.; Guo, H.; Pan, W. Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J. Pharm. Pharmacol. 2006, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Valerie, J.S. Antibacterial free fatty acids: Activities Mechanism of action and Biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharate, S.S.; Vishwakarma, R.A. Thermodynamic equilibrium solubility measurements in simulated fluids by 96-well plate method in early drug discovery. Bioorg. Med. Chem. Lett. 2015, 25, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, T.; Nocker, P.; Lavi, G.; Kuhlmann, J.; Czermak, P.; Runkel, F. Development of an alternative, time and cost saving method of creating pseudoternary diagrams using the example of a microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 187–192. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Formulation and evaluation of Microemulsion based Drug Delivery system for intra nasal administration of Olanzapine. Int. J. Biomed. Pharm. Sci. 2012, 7, 20–27. [Google Scholar]
- European Medicines Agency. Stability Testing on New Drug Substances and Products. In Proceedings of the International Conference on Harmonization Guidance for Industry Q1A (R2); Geneva International Federation of Pharmaceutical Manufacturers & Associations: Geneva, Switzerland, 2000. [Google Scholar]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Alany, R.G.; Rades, T.; Nicoll, J.; Tucker, I.G.; Davies, N.M. W/O microemulsions for ocular delivery: Evaluation of ocular irritation and precorneal retention. J. Control. Release 2006, 111, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]










| Formulation | Composition | Particle Size ± SD (nm) | PDI ± SD | Zeta Potential (mV) | % Transmittance (at 600 nm) | pH | Viscosity (mPa∙S) | α-Linolenic Acid Content % | Contact Angle on Hydrophilic Surface (°) | Contact Angle on Hydrophobic Surface (°) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid (FA)% | S/CoS% | Water% | ||||||||||
| T1 (Tween80/Transcutol P) | 4 | 88 | 8 | 190.4 ± 2.3 | 0.309 ± 0.12 | 0.124 ± 0.022 | 98% | 5.96 ± 0.02 | 65.32 ± 2.15 | 92.5 | 14.1 ± 0.85 | 29.5 ± 2.43 |
| T2 (Tween8/Transcutol P) | 6 | 60 | 34 | 205.1 ± 1.2 | 0.551 ± 0.085 | 0.107 ± 0.014 | 98% | 4.66 ± 0.01 | 96.12 ± 4.22 | 95.9 | 12.2 ± 0.06 | 35.2 ± 3.73 |
| T3 (Tween8/Transcutol P) | 35 | 60 | 5 | 219.1 ± 1.5 | 0.383 ± 0.056 | 0.394 ± 0.035 | 115% | 5.33 ± 0.02 | 56.67 ± 2.32 | 99.8 | 15.7 ± 2.51 | 25.9 ± 3.70 |
| C1 (Cremophor EL/Transcutol P) | 4 | 86 | 10 | 340.1 ± 1.9 | 0.561 ± 0.032 | 0.025 ± 0.011 | 99% | 6.23 ± 0.02 | 82.53 ± 1.63 | 91.8 | 25.1 ± 2.41 | 34.8 ± 3.08 |
| C2 (Cremophor EL/Transcutol P) | 6 | 62 | 32 | 225.6 ± 2.1 | 0.392 ± 0.14 | 0.303 ± 0.054 | 98% | 5.43 ± 0.01 | 101.42 ± 3.11 | 93.3 | 16.2 ± 3.36 | 43.8 ± 4.30 |
| C3 (Cremophor EL/Transcutol P) | 35 | 60 | 5 | 246.4 ± 3.3 | 0.484 ± 0.025 | 0.102 ± 0.012 | 97% | 5.47 ± 0.02 | 74.46 ± 2.56 | 96.4 | 25.2 ± 2.60 | 38.0 ± 5.68 |
| Formulation | Zone of Inhibition against N. gonorrhoeae | Zone of Inhibition against S. aureus |
|---|---|---|
| T1 | 6.5 ± 0.7 mm | 14.5 ± 0.7 mm |
| T2 | 8.5 ± 0.7 mm | 15 ± 1.00 mm |
| T3 | 22 ± 1.00 mm | 21.5 ± 0.7 mm |
| C1 | 6.75 ± 0.4 mm | 17.75 ± 0.4 mm |
| C2 | 8 ± 1.00 mm | 16.2 ± 0.8 mm |
| C3 | 22.75 ± 0.4 mm | 22.25 ± 1.06 mm |
| Active ingredients | ||
| Tween 80 | 0.00 | 16.0 ± 1.4 mm |
| Cremophor–EL | 0.00 | 11.5 ± 0.7 mm |
| Transcutol P | 0.00 | 7.5 ± 0.7 mm |
| α-linolenic acid (1 mM) | 10.2 ± 0.6 | 7.5 ± 0.6 |
| Opacity | Score | Fluorescein Permeability | Score | Cumulative Score | Interpretation |
|---|---|---|---|---|---|
| None | 0 | None | 0 | ≤0.5 | None |
| Slight | 1 | Diffuse and weak | 0.5 | 0.6–1.9 | Slight |
| Marked | 2 | Confluent and weak | 1 | 2.0–4.0 | Moderate |
| Severe | 3 | Confluent and intense | 1.5 | >4 | Severe |
| Opaque | 4 | - | - | - | - |
| Score | Cumulative Score | Irritation Assessment | |||
|---|---|---|---|---|---|
| Effect | 0.5 min | 2 min | 5 min | 0–0.9 | None |
| Hyperaemia | 5 | 3 | 1 | 1.0–4.9 | Slight |
| Haemorrhage | 7 | 5 | 3 | 5.0–8.9 | Moderate |
| Coagulation | 9 | 7 | 5 | 9.0–21.0 | Severe |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, U.; ElShaer, A.; Snyder, L.A.S.; Al-Kinani, A.A.; Le Gresley, A.; Alany, R.G. Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials 2018, 8, 51. https://doi.org/10.3390/nano8010051
Butt U, ElShaer A, Snyder LAS, Al-Kinani AA, Le Gresley A, Alany RG. Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials. 2018; 8(1):51. https://doi.org/10.3390/nano8010051
Chicago/Turabian StyleButt, Ummara, Amr ElShaer, Lori A. S. Snyder, Ali A. Al-Kinani, Adam Le Gresley, and Raid G. Alany. 2018. "Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus" Nanomaterials 8, no. 1: 51. https://doi.org/10.3390/nano8010051
APA StyleButt, U., ElShaer, A., Snyder, L. A. S., Al-Kinani, A. A., Le Gresley, A., & Alany, R. G. (2018). Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials, 8(1), 51. https://doi.org/10.3390/nano8010051