Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
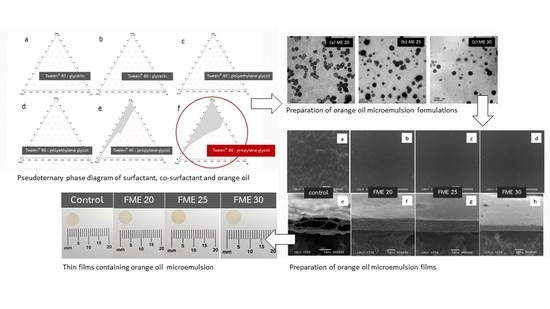
2.2. Preparation and Optimization of Orange Oil ME Formulations
2.2.1. Screening and Selection of Surfactants
2.2.2. Screening and Selection of Co-Surfactants
2.2.3. Influence of Surfactant and Co-Surfactant Mass Ratio on ME Formation
2.2.4. Preparation of ME Formulation
2.2.5. Morphology and Vesicle Size Determination of ME
2.3. Preparation and Characterization of Orange Oil ME-Loaded Pectin Thin Film
2.3.1. Film Preparation
2.3.2. Morphological Characterization of the Films
2.3.3. Film Thickness
2.3.4. Testing of Tensile Properties
2.3.5. Limonene Loading Content in Pectin Thin Film
2.4. Antibacterial Activity of Orange Oil ME-Loaded Pectin Thin Film
2.5. Statistical Analysis
3. Results and Discussion
3.1. ME Preparation and Characterization
3.1.1. ME Preparation
3.1.2. ME Characterization
3.2. Film Characterization
3.2.1. Thin Film Morphology
3.2.2. Mechanical Properties
3.2.3. Limonene Loading Content
3.3. Antibacterial Activity of Orange Oil ME-Loaded Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavor encapsulation and controlled release-a review. Int. J. Food. Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Andrade, L.R.; Farina, M.; Rocha-Leao, M.H.M. Characterization of short chain fatty acid microcapsules produced by spray drying. Mater. Sci. Eng. C. 2004, 24, 653–658. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; Cellier, J.; Duke, P.A.K. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: London, UK, 2002. [Google Scholar]
- Mercier, D.; Knevitt, A. Using topical aromatherapy for the management of fungating wounds in a palliative care unit. J. Wound Care. 2005, 14, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Obidi, O.F.; Adelowotan, A.O.; Ayoola, G.A.; Johnson, O.O.; Hassan, M.O.; Nwachukwu, S.C.U. Antimicrobial activity of orange oil on selected pathogens. Int. J. Biotechnol. 2013, 2, 113–122. [Google Scholar]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Magwa, M.L.; Gundidza, M.; Gweru, N. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin-a review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Kantaria, S.; Rees, G.D.; Lawrence, M.J. Formulation of electrically conducting microemulsion based organogels. Int. J. Pharm. 2003, 250, 65–83. [Google Scholar] [CrossRef]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Ther. Carr. Sys. 1999, 16, 461–521. [Google Scholar] [CrossRef]
- Soukharev, A.R.; Wojciechowska, J. Microemulsions as potential ocular drug delivery systems: Phase diagrams and physical properties depending on ingredients. Acta Pol. Pharm. 2005, 62, 465–471. [Google Scholar]
- Flanagan, J.; Singh, H. Microemulsions: A potential delivery system for bioactives in food. Crit. Rev. Food Sci. Nutr. 2006, 46, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Leser, M.E.; Sagalowicz, L.; Michel, M.; Watzke, H.J. Self-assembly of polar food lipids. Adv. Colloid Interface Sci. 2006, 123, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.R.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Hashtjin, A.M.; Soleiman, A. Optimization of ultrasonic emulsification conditions for the production of orange peel essential oil nanoemulsions. J. Food Sci. Technol. 2015, 52, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Yotsawimonwat, S.; Okonoki, S.; Krauel, K.; Sirithunyalug, J.; Sirithunyalug, B.; Rades, T. Characterisation of microemulsions containing orange oil with water and propylene glycol as hydrophilic components. Pharmazie 2006, 61, 920–926. [Google Scholar] [PubMed]
- Azeem, A.; Khan, Z.I.; Aqil, M.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev. Ind. Pharm. 2009, 35, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Nagarsenker, M.S. Design and evaluation of microemulsions for improved parenteral delivery of propofol. AAPS Pharm. Sci. Tech. 2008, 9, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS Pharm. Sci. Tech. 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, H.C.; Chambin, O. Effect of plasticizer type on tensile property and in vitro indomethacin release of thin films based on low-methoxyl pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef]
- Lee, T.W.; Kim, J.C.; Hwang, S.J. Hydrogel patches containing triclosan for acne treatment. Eur. J. Pharm. Biopharm. 2003, 6, 407–412. [Google Scholar] [CrossRef]
- Brantner, A.; Pfeiffer, K.P.; Brantner, H. Applicability of diffusion methods required by the pharmacopoeias for testing anti-bacterial activity of natural compounds. Pharmazie 1994, 49, 512–516. [Google Scholar] [PubMed]
- Lawrence, J. Surfactant systems: Microemulsions and vesicles as vehicles for drug delivery. Eur. J. Drug Metab. Pharmacokinet. 1994, 19, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Goindi, S.; Khurana, R. Formulation, characterization and evaluation of an optimized microemulsion formulation of griseofulvin for topical application. Colloids Surf. B 2013, 105, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf. B 2010, 76, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.K.; Pathak, K. Wet process-induced phase-transited drug delivery system: A means for achieving osmotic, controlled, and level A IVIVC for poorly water-soluble drug. Drug Dev. Ind. Pharm. 2008, 34, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Weisan, P.; Jiayu, L.; Ying, Z. Preparation, evaluation, and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev. Ind. Pharm. 2004, 30, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.W. Mixing performance of various geometries-emulsification perspective. Procedia Food Sci. 2011, 1, 131–137. [Google Scholar] [CrossRef]
- Schultz, S.; Wagner, G.; Urban, K.; Ulrich, J. High-pressure homogenization as a process for emulsion formation. Chem. Eng. Technol. 2004, 27, 361–368. [Google Scholar] [CrossRef]
- Tadros, T. Principles of emulsion stabilization with special reference to polymeric surfactants. J. Cosmet. Sci. 2006, 57, 153–169. [Google Scholar] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and anti-bacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, R.N.; Skurtys, O.; Osorio, F.; Aguilera, J.M.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sáinz, C.; Avena-Bustillos, R.J.; Wood, D.F.; Williams, T.G.; McHugh, T.H. Nanoemulsions prepared by a low-energy emulsification method applied to edible films. J. Agric. Food Chem. 2010, 58, 11932–11938. [Google Scholar] [CrossRef] [PubMed]
- Quezada Gallo, J.A.; Debeaufort, F.; Callegarin, F.; Voilley, A. Lipid hydrophobicity, physical state and distribution effects on the properties of emulsion-based edible films J. Membr. Sci. 2000, 180, 37–46. [Google Scholar] [CrossRef]
- Otoni, C.G.; Pontes, S.F.; Medeiros, E.A.; Soares Nde, F. Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, 107–116. [Google Scholar] [CrossRef]




| Microemulsion | Orange Oil (%) | Smix (%) | Water (%) | Transmittance ± SD (%) | Droplet Size ± SD (nm) | PdI ± SD |
|---|---|---|---|---|---|---|
| ME 20 | 20 | 70 | 10 | 98.52 ± 0.02 a | 73.42 ± 1.60 a | 0.216 ± 0.03 a |
| ME 25 | 25 | 65 | 10 | 97.51 ± 0.03 b | 75.17 ± 7.27 a | 0.224 ± 0.03 a |
| ME 30 | 30 | 60 | 10 | 96.18 ± 0.01 b | 77.63 ± 0.57 a | 0.223 ± 0.05 a |
| Film | Thickness ± SD (mm) | Tensile Strength ± SD (MPa) | Elongation ± SD (%) | Young’s Modulus ± SD (MPa) | Loading Content (%) |
|---|---|---|---|---|---|
| Control | 0.089 ± 0.009 a | 7.28 ± 0.85 a | 5.62 ± 0.46 a | 129.33 ± 6.83 a | 60.75 ± 3.11 a |
| FME 20 | 0.094 ± 0.021 b | 2.28 ± 0.86 b | 7.60 ± 3.01 b | 30.52 ± 4.59 b | 83.24 ± 5.25 b |
| FME 25 | 0.093 ± 0.010 b | 3.63 ± 1.36 b | 9.29 ± 3.04 b | 33.74 ± 3.61 b | 83.88 ± 2.43 b |
| FME 30 | 0.083 ± 0.017 a | 3.24 ± 0.91 b | 9.75 ± 0.90 b | 34.74 ± 9.01 b | 85.10 ± 2.01b |
| Sample | Average Diameter of Inhibition Zones (mm) ± SD | |
|---|---|---|
| S. aureus | P. acnes | |
| FME 20 | ND | 13.64 ± 0.25 |
| FME 25 | ND | 15.18 ± 0.09 |
| FME 30 | 8.32 ± 0.11 | 16.10 ± 0.02 |
| Control film | ND | ND |
| Tween 80 | ND | ND |
| Propylene glycol | ND | ND |
| Ampicillin (10 mg/mL) | 32.87 ± 0.96 | 19.43 ± 0.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.-M.; Park, E.-S. Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials 2018, 8, 545. https://doi.org/10.3390/nano8070545
Jantrawut P, Boonsermsukcharoen K, Thipnan K, Chaiwarit T, Hwang K-M, Park E-S. Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials. 2018; 8(7):545. https://doi.org/10.3390/nano8070545
Chicago/Turabian StyleJantrawut, Pensak, Kasidech Boonsermsukcharoen, Kanyanut Thipnan, Tanpong Chaiwarit, Kyu-Mok Hwang, and Eun-Seok Park. 2018. "Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion" Nanomaterials 8, no. 7: 545. https://doi.org/10.3390/nano8070545
APA StyleJantrawut, P., Boonsermsukcharoen, K., Thipnan, K., Chaiwarit, T., Hwang, K. -M., & Park, E. -S. (2018). Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials, 8(7), 545. https://doi.org/10.3390/nano8070545