Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications
Abstract
:1. Introduction
2. Thermosensitive Nanomaterials: Synthesis, Properties and Functionalization
2.1. Physical Mechanism: Critical Solution Behavior
2.2. Synthesis of Thermoresponsive Polymeric Particles
2.2.1. Homogeneous Nucleation
2.2.2. Emulsification
2.2.3. Complexation
2.3. Hybrid Particles: Incorporation of Magnetic Field and/or Infrared Radiation Sensitivity
2.3.1. Magnetic Field: Iron Oxides
2.3.2. Infrared Radiation: Gold
2.3.3. Functionalization
2.4. Properties
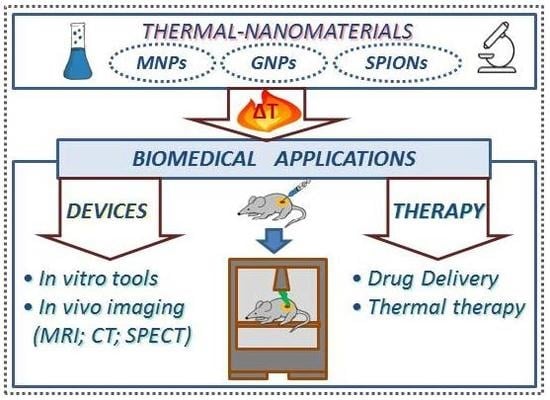
3. Biomedical Applications of Thermal-Nanomaterials
3.1. Analytical and Diagnostic Devices
3.1.1. Thermal-Nanomaterials as Diagnostic Devices
3.1.2. Thermal-Nanomaterials as Current Imaging Tests Enhancers
3.2. Thermo-Sensitive Cargo Delivery
3.2.1. Liposomes
3.2.2. Micelles
3.2.3. Core-Shell Nanodevices
3.2.4. Hydrogels
3.2.5. Polymersomes
3.3. Thermal Therapy
3.4. Theranostics
4. Future Challenges and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, C.R.; Fernandez, L.E.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; Nel, A.E.; Penner, R.M.; Willson, C.G.; Weiss, P.S. Nano Day: Celebrating the Next Decade of Nanoscience and Nanotechnology. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Prato, M. Nanomaterials for (Nano)medicine. ACS Med. Chem. Lett. 2013, 4, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodriguez-Serrano, F.; Peran, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- De Mello Donegá, C. Nanoparticles: Workhorses of Nanoscience; Springer: Berlin/Heidelberg, Germany, 2014; pp. 5–7. [Google Scholar]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef] [PubMed]
- Mele, E. Introduction. Smart materials in biomedicine In Smart Nanoparticles for Biomedicine; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 1–13. [Google Scholar]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2018, 19, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J.S. Future Directions in Minimally Invasive Intervention. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 346–352. [Google Scholar] [CrossRef]
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, N.; Guo, Z.Y. Non-Fourier heat conductions in nanomaterials. J. Appl. Phys. 2011, 110, 064310. [Google Scholar] [CrossRef]
- Ashby, M.F.; Ferreira, P.J.; Schodek, D.L. Nanomaterials: Properties. In Nanomaterials, Nanotechnologies and Design; Elsevier: Amsterdam, The Netherlands, 2009; pp. 199–255. [Google Scholar]
- Sahle, F.F.; Gulfam, M.; Lowe, T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2018, 23, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Justin, C.; Philip, S.A.; Samrot, A.V. Synthesis and characterization of superparamagnetic iron-oxide nanoparticles (SPIONs) and utilization of SPIONs in X-ray imaging. Appl. Nanosci. 2017, 7, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Ciocca, D.R. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int. J. Hyperth. 2008, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordonez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Thermoresponsive magnetic colloids. Colloid Polym. Sci. 2007, 285, 953–966. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Hocine, S.; Li, M.H. Thermoresponsive self-assembled polymer colloids in water. Soft Matter 2013, 9, 5839–5861. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S. Thermodynamically Stable Globule State of a Single Poly(N-isopropylacrylamide) Chain in Water. Macromolecules 1995, 28, 5388–5390. [Google Scholar] [CrossRef]
- Chen, J.; Pei, Y.; Yang, L.-M.; Shi, L.-L.; Luo, H.-J. Synthesis and Properties of Poly(N-isopropylacrylamide-co-acrylamide) Hydrogels. Macromol. Symp. 2005, 225, 103–112. [Google Scholar] [CrossRef]
- Ankareddi, I.; Brazel, C.S. Synthesis and characterization of grafted thermosensitive hydrogels for heating activated controlled release. Int. J. Pharm. 2007, 336, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hertle, Y.; Hellweg, T. Thermoresponsive copolymer microgels. J. Mater. Chem. B 2013, 1, 5874–5885. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Hu, S.-H.; Liu, D.-M.; Chen, S.-Y.; Chen, I.W. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today 2009, 4, 52–65. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.; Chen, J.; Cheung, C.; Chilkoti, A.; Ding, Z.; Dong, L.; Fong, R.; et al. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586. [Google Scholar] [CrossRef]
- Yokoyama, M. Gene delivery using temperature-responsive polymeric carriers. Drug Discov. Today 2002, 7, 426–432. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Pelton, R.; Hoare, T. Microgels and Their Synthesis: An Introduction. Microgel Suspens. 2011. [Google Scholar] [CrossRef]
- McPhee, W.; Tam, K.; Pelton, R. Poly(N-Isopropylacrylamide) lattices prepared with sodium dodecyl sulfate. J. Colloid Interface Sci. 1993, 156, 24–30. [Google Scholar] [CrossRef]
- Goodwin, J.W.; Hearn, J.; Ho, C.C.; Ottewill, R.H. The preparation and characterisation of polymer latices formed in the absence of surface active agents. Br. Polym. J. 1973, 5, 347–362. [Google Scholar] [CrossRef]
- Kuckling, D.; Vo, C.D.; Wohlrab, S.E. Preparation of nanogels with temperature-responsive core and pH-responsive arms by photo-cross-linking. Langmuir 2002, 18, 4263–4269. [Google Scholar] [CrossRef]
- Shen, H.W.; Eisenberg, A. Block length dependence of morphological phase diagrams of the ternary system of PS-b-PAA/dioxane/H2O. Macromolecules 2000, 33, 2561–2572. [Google Scholar] [CrossRef]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Chern, C.S. Principles and Applications of Emulsion Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Landfester, K.; Willert, M.; Antonietti, M. Preparation of Polymer Particles in Nonaqueous Direct and Inverse Miniemulsions. Macromolecules 2000, 33, 2370–2376. [Google Scholar] [CrossRef]
- Chen, L.-W.; Yang, B.-Z.; Wu, M.-L. Synthesis and kinetics of microgel in inverse emulsion polymerization of acrylamide. Prog. Org. Coat. 1997, 31, 393–399. [Google Scholar] [CrossRef]
- Dowding, P.J.; Vincent, B.; Williams, E. Preparation and Swelling Properties of Poly(NIPAM) “Minigel” Particles Prepared by Inverse Suspension Polymerization. J. Colloid Interface Sci. 2000, 221, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Kumacheva, E.; Garstecki, P. Microfluidic Reactors for Polymer Particles; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Feng, X.; Pelton, R.; Leduc, M.; Champ, S. Colloidal Complexes from Poly(vinyl amine) and Carboxymethyl Cellulose Mixtures. Langmuir 2007, 23, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Gan, D.J.; Serpe, M.J.; Lyon, L.A. Hollow thermoresponsive microgels. Small 2005, 1, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Ballauff, M.; Lu, Y. “Smart” nanoparticles: Preparation, characterization and applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Gan, D.J.; Lyon, L.A. Tunable swelling kinetics in core-shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001, 123, 7511–7517. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Retama, J.; Zafeiropoulos, N.E.; Serafinelli, C.; Rojas-Reyna, R.; Voit, B.; Cabarcos, E.L.; Stamm, M. Synthesis and characterization of thermosensitive PNIPAM microgels covered with superparamagnetic gamma-Fe2O3 nanoparticles. Langmuir 2007, 23, 10280–10285. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Perez-Juste, J.; Hellweg, T.; Liz-Marzan, L.M. Nanorod-coated PNIPAM microgels: thermoresponsive optical properties. Small 2007, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Xu, S.Q.; Kumacheva, E. Polymer microgels: Reactors for semiconductor, metal, and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Zhang, H.; Kumacheva, E. Microgels: Old materials with new applications. Ann. Rev. of Mat. Res. 2006, 36, 117–142. [Google Scholar] [CrossRef]
- Suzuki, D.; McGrath, J.G.; Kawaguchi, H.; Lyon, L.A. Colloidal crystals of thermosensitive, core/shell hybrid microgels. J. Phys. Chem. C 2007, 111, 5667–5672. [Google Scholar] [CrossRef]
- Kondo, A.; Kamura, H.; Higashitani, K. Development and Application of Thermosensitive Magnetic Immunomicrospheres for Antibody Purification. App. Microbiol. Biotech. 1994, 41, 99–105. [Google Scholar] [CrossRef]
- Singh, N.; Lyon, L.A. Au nanoparticle templated synthesis of pNIPAm nanogels. Chem. Mat. 2007, 19, 719–726. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.E.; Seip, C.T.; O’Connor, C.J. Magnetism of nanophase metal and metal alloy particles formed in ordered phases. J. Appl. Phys. 1999, 85, 5184–5186. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- LesliePelecky, D.L.; Rieke, R.D. Magnetic properties of nanostructured materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khan, S.R.; Begum, R.; Ijaz, A. Review on synthesis, properties, characterization, and applications of responsive microgels fabricated with gold nanostructures. Rev. Chem. Eng. 2016, 32, 49–69. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, K.; Guan, Y.; Zhang, Y.J. Assembling of gold nanorods on P(NIPAM-AAPBA) microgels: A large shift in the plasmon band and colorimetric glucose sensing. RSC Adv. 2012, 2, 4768–4776. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, L.; Wang, T.; Wang, Q.M.; Gao, Y.; Wang, N. Poly(N-isopropylacrylamide)-Au hybrid microgels: Synthesis, characterization, thermally tunable optical and catalytic properties. Soft Matter 2013, 9, 10966–10970. [Google Scholar] [CrossRef]
- Echeverria, C.; Mijangos, C. Effect of Gold Nanoparticles on the Thermosensitivity, Morphology, and Optical Properties of Poly(acrylamide-acrylic acid) Microgels. Macromol. Rapid Commun. 2010, 31, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Raula, J.; Shan, J.; Nuopponen, M.; Niskanen, A.; Jiang, H.; Kauppinen, E.I.; Tenhu, H. Synthesis of gold nanoparticles grafted with a thermoresponsive polymer by surface-induced reversible-addition-fragmentation chain-transfer polymerization. Langmuir 2003, 19, 3499–3504. [Google Scholar] [CrossRef]
- Wang, C.; Flynn, N.T.; Langer, R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv. Mater. 2004, 16, 1074–1079. [Google Scholar] [CrossRef]
- Suzuki, D.; Nagase, Y.; Kureha, T.; Sato, T. `Internal Structures of Thermosensitive Hybrid Microgels Investigated by Means of Small-Angle X-ray Scattering. J. Phys. Chem. B 2014, 118, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, Q.M.; Wang, T.; Ren, S.P.; Gao, Y.; Wang, N. Thermo-, pH-, and Light-Responsive Poly(N-isopropylacrylamide-co-methacrylic acid)-Au Hybrid Microgels Prepared by the in Situ Reduction Method Based on Au-Thiol Chemistry. J. Phys. Chem. B 2014, 118, 7177–7186. [Google Scholar] [CrossRef] [PubMed]
- Jalili, N.A.; Muscarello, M.; Gaharwar, A.K. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng. Transl. Med. 2016, 1, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Brazel, C.S. Magnetothermally-responsive Nanomaterials: Combining Magnetic Nanostructures and Thermally-Sensitive Polymers for Triggered Drug Release. Pharm. Res.-Dordr. 2009, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Tanaka, T.; Sato, E.; Hirokawa, Y.; Hirotsu, S.; Peetermans, J. Critical Kinetics of Volume Phase-Transition of Gels. Phys. Rev. Lett. 1985, 55, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.C.; Hearn, J.; Steward, P.A. The cleaning of polymer colloids. Adv. Colloid Interface Sci. 1999, 81, 77–165. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: Cambridge, MA, USA, 1981; pp. 59–124. [Google Scholar]
- Nielsen, O.S.; Horsman, M.; Overgaard, J. A future for hyperthermia in cancer treatment? Eur. J. Cancer 2001, 37, 1587–1589. [Google Scholar] [CrossRef]
- Mertz, D.; Sandre, O.; Begin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta 2017, 1861, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayo, L.L. Stimuli-responsive nanocarriers for intracellular delivery. Biophys. Rev. 2017, 9, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avella-Oliver, M.; Morais, S.; Puchades, R.; Maquieira, A. Towards photochromic and thermochromic biosensing. TrAC-Trends Anal. Chem. 2016, 79, 37–45. [Google Scholar] [CrossRef]
- Harrington, W.N.; Haji, M.R.; Galanzha, E.I.; Nedosekin, D.A.; Nima, Z.A.; Watanabe, F.; Ghosh, A.; Biris, A.S.; Zharov, V.P. Photoswitchable non-fluorescent thermochromic dye-nanoparticle hybrid probes. Sci. Rep. 2016, 6, 36417. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Guye, K.N.; Masiello, D.J.; Ginger, D.S. Dynamic Optical Switching of Polymer/Plasmonic Nanoparticle Hybrids with Sparse Loading. J. Phys. Chem. B 2017, 121, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, C.; Polavarapu, L.; Solis, D.M.; Taboada, J.M.; Obelleiro, F.; Contreras-Caceres, R.; Pastoriza-Santos, I.; Perez-Juste, J. Gold Nanorod-pNIPAM Hybrids with Reversible Plasmon Coupling: Synthesis, Modeling, and SERS Properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Manikas, A.C.; Romeo, G.; Papa, A.; Netti, P.A. Highly efficient surface-enhanced Raman scattering substrate formulation by self-assembled gold nanoparticles physisorbed on poly(N-isopropylacrylamide) thermoresponsive hydrogels. Langmuir 2014, 30, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Rudrum, A.W.; Herrmann, L.O.; Turek, V.; Baumberg, J.J. Polymer-assisted self-assembly of gold nanoparticle monolayers and their dynamical switching. Nanoscale 2016, 8, 15864–15869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.L.; Cao, F.H.; Wang, J.L.; Yu, Z.L.; Ge, J.; Lu, Y.; Wang, Z.H.; Yu, S.H. Highly Stimuli-Responsive Au Nanorods/Poly(N-isopropylacrylamide) (PNIPAM) Composite Hydrogel for Smart Switch. ACS Appl. Mater. Interfaces 2017, 9, 24857–24863. [Google Scholar] [CrossRef] [PubMed]
- Hembury, M.; Beztsinna, N.; Asadi, H.; van den Dikkenberg, J.B.; Meeldijk, J.D.; Hennink, W.E.; Vermonden, T. Luminescent Gold Nanocluster-Decorated Polymeric Hybrid Particles with Assembly-Induced Emission. Biomacromolecules 2018, 19, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, L.; Cai, Z.H.; Xu, J.W.; Xu, Z.; Zhang, D.; Hu, X.B. Plasmonic nanoparticles tuned thermal sensitive photonic polymer for biomimetic chameleon. Sci. Rep. 2016, 6, 31328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Ma, N.; Tong, L.Y.; He, F.; Li, L.D. Control of Metal-Enhanced Fluorescence with pH- and Thermoresponsive Hybrid Microgels. Langmuir 2012, 28, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Samai, S.; Qian, Z.; Ling, J.; Guye, K.N.; Ginger, D.S. Optical Properties of Reconfigurable Polymer/Silver Nanoprism Hybrids: Tunable Color and Infrared Scattering Contrast. ACS Appl. Mater. Interfaces 2018, 10, 8976–8984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, C.; Yang, X.D.; Shen, B.W.; Sun, Y.Q.; Chen, Y.; Xu, X.W.; Sun, H.C.; Yu, K.; Yang, B.; et al. pH- and Temperature-Sensitive Hydrogel Nanoparticles with Dual Photoluminescence for Bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Schuerle, S.; Dudani, J.S.; Christiansen, M.G.; Anikeeva, P.; Bhatia, S.N. Magnetically Actuated Protease Sensors for in Vivo Tumor Profiling. Nano Lett. 2016, 16, 6303–6310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Beauregard, D.A.; Loizou, L.; Davletov, B.; Brindle, K.M. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nat. Med. 2001, 7, 1241. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, X.; Jiang, J.; Guo, G.; Wu, H.; Wu, M.; Zhu, H. Stem cell tracking using effective self-assembled peptide-modified superparamagnetic nanoparticles. Nanoscale 2018, 10, 15967–15979. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Zhou, X.Y.; Yu, E.; Zheng, B.; Conolly, S. In vivo tracking and quantification of inhaled aerosol using magnetic particle imaging towards inhaled therapeutic monitoring. Theranostics 2018, 8, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Shi, X. Acetylated Polyethylenimine-Entrapped Gold Nanoparticles Enable Negative Computed Tomography Imaging of Orthotopic Hepatic Carcinoma. Langmuir 2018, 34, 8701–8707. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- ThermoDox® Enhancing the Efficacy of Doxorubicin with Heat-Activated Liposome Technology. Available online: http://celsion.com/thermodox/ (accessed on 9 October 2018).
- Dunne, M.; Dou, Y.N.; Drake, D.M.; Spence, T.; Gontijo, S.M.L.; Wells, P.G.; Allen, C. Hyperthermia-mediated drug delivery induces biological effects at the tumor and molecular levels that improve cisplatin efficacy in triple negative breast cancer. J. Control. Release 2018, 282, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lee, H.S.; Jung, J.H.; Kim, H.K.; Park, J.H. Photothermally Amplified Therapeutic Liposomes for Effective Combination Treatment of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.Q.; Xu, C.R.; Zhao, X.M.; Lin, C.S.; Yang, X.; Xin, X.F.; Zhang, L.; Qn, C.; Han, X.P.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Zangabad, P.S.; Aghanejad, A.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr. Polym. 2017, 172, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.L.; Chen, Y.I.; Liu, H.J.; Lee, P.C.; Luo, T.Y.; Shieh, M.J. A novel temperature-responsive micelle for enhancing combination therapy. Int. J. Nanomed. 2016, 11, 3357–3369. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Zangabad, P.S.; Barar, J.; Aghanejad, A.; Erfan-Niya, H.; Omidi, Y. Thermo-sensitive chitosan copolymer-gold hybrid nanoparticles as a nanocarrier for delivery of erlotinib. Int. J. Biol. Macromol. 2018, 106, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, A.; Wang, W.; Zurakowski, D.; Langer, R.S.; Kohane, D.S. Photothermally targeted thermosensitive polymer-masked nanoparticles. Nano Lett. 2014, 14, 3697–3701. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.J.; Wang, Y.F.; Sun, J. Encapsulating magnetic and fluorescent mesoporous silica into thermosensitive chitosan microspheres for cell imaging and controlled drug release in vitro. Colloid Surf. B 2014, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Irie, K. Synthesis of temperature-dependent elastin-like peptide-modified dendrimer for drug delivery. Biopolymers 2013, 100, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Zhao, Y.N.; Zhao, J.; Han, M.H.; Zhang, A.F.; Wang, X.T. Codendrimer from Polyamidoamine (PAMAM) and Oligoethylene Dendron as a Thermosensitive Drug Carrier. Bioconjug. Chem. 2014, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Hassan, M.A.; Ahmed, I.S.; Shamma, R. Thermogelling Platform for Baicalin Delivery for Versatile Biomedical Applications. Mol. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C-Mater. 2018, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, J.; Cai, P.; Xiao, H. Dual-responsive IPN hydrogel based on sugarcane bagasse cellulose as drug carrier. Int. J. Biol. Macromol. 2018, 118, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Chavez, E.; Tsai, K.L.; Yang, K.C.; Kuo, W.T.; Yang, Y.P.; Chiou, S.H.; Lin, F.H. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr. Polym. 2017, 173, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Sirianni, P.; Di Rienzo, A.M.; Chiono, V. Thermosensitive block copolymer hydrogels based on poly(varepsilon-caprolactone) and polyethylene glycol for biomedical applications: State of the art and future perspectives. J. Biomed. Mater. Res. Part A 2015, 103, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.L.; Zheng, S.X. Poly(N-isopropylacrylamide)-block-poly(acrylic acid) hydrogels: Synthesis and rapid thermoresponsive properties. Colloid Polym. Sci. 2014, 292, 2633–2645. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Y.L.; Qu, Y.; Liao, J.F.; Chu, B.Y.; Zhang, H.P.; Luo, F.; Qian, Z.Y. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016, 6, 19077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kuo, C.Y.; Chen, S.H.; Mao, S.H.; Chang, C.Y.; Shalumon, K.T.; Chen, J.P. Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. Int. J. Mol. Sci. 2018, 19, 1373. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Qi, T.; Liao, J.; Chu, B.; Yang, Q.; Li, W.; Qu, Y.; Luo, F.; Qian, Z. Controlled release of cisplatin from pH-thermal dual responsive nanogels. Biomaterials 2013, 34, 8726–8740. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Sun, P.P.; Wu, A.L.; Qiao, X.X.; Lu, F.; Zheng, L.Q. Facile fabrication of thermo/redox responsive hydrogels based on a dual crosslinked matrix for a smart on-off switch. Soft Matter 2018, 14, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Chao, Y.; Dong, Z.; Yi, X.; He, J.; Liu, Z.; Yang, K. Development of a thermosensitive protein conjugated nanogel for enhanced radio-chemotherapy of cancer. Nanoscale 2018. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Yealland, G.; Honzke, S.; Edlich, A.; Geisendorfer, B.; Kleuser, B.; Hedtrich, S.; Calderon, M. Breaking the Barrier—Potent Anti-Inflammatory Activity following Efficient Topical Delivery of Etanercept using Thermoresponsive Nanogels. Theranostics 2018, 8, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Shih, M.H.; Hsu, W.B.; Dubey, N.K.; Lee, W.F.; Lin, T.Y.; Hsieh, M.Y.; Chen, C.F.; Peng, K.T.; Huang, T.J.; et al. Evaluation of a novel biodegradable thermosensitive keto-hydrogel for improving postoperative pain in a rat model. PLoS ONE 2017, 12, e0186784. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, X.; Wen, Y.T.; Zhu, J.L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Song, H.J.; Zhang, Y.M.; Liu, J.J.; Cheng, Z.; Liang, X.J.; Wang, W.W.; Kong, D.L.; Liu, J.F. FRET-enabled monitoring of the thermosensitive nanoscale assembly of polymeric micelles into macroscale hydrogel and sequential cognate micelles release. Biomaterials 2017, 145, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Du, X.; Feng, G.; Zhang, Y.; Li, J.; Lin, B.; Yang, L.; Fu, S.; Wu, J. Efficient inhibition of cervical cancer by dual drugs loaded in biodegradable thermosensitive hydrogel composites. Oncotarget 2018, 9, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Lin, H.W.; Jiang, D.P.; Xu, G.F.; Fang, X.L.; He, L.; Xu, M.S.; Tang, B.Q.; Wang, Z.Y.; Cui, D.X.; et al. Co-delivery of VEGF and bFGF via a PLGA nanoparticle-modified BAM for effective contracture inhibition of regenerated bladder tissue in rabbits. Sci. Rep. 2016, 6, 20784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezazadeh, M.; Parandeh, M.; Akbari, V.; Ebrahimi, Z.; Taheri, A. Incorporation of rosuvastatin-loaded chitosan/chondroitin sulfate nanoparticles into a thermosensitive hydrogel for bone tissue engineering: Preparation, characterization, and cellular behavior. Pharm. Dev. Technol. 2018, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.S.; Park, H.; Lee, J.; Park, W.H. Injectable methylcellulose hydrogel containing calcium phosphate nanoparticles for bone regeneration. Int. J. Biol. Macromol. 2018, 109, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Brahima, S.; Boztepe, C.; Kunkul, A.; Yuceer, M. Modeling of drug release behavior of pH and temperature sensitive poly(NIPAAm-co-AAc) IPN hydrogels using response surface methodology and artificial neural networks. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Zhao, Y.; Xie, H.; Lin, Q.; He, Z.; Wang, X.; Li, J.; Zhang, H.; Wang, C.; et al. A Thermosensitive Heparin-Poloxamer Hydrogel Bridges aFGF to Treat Spinal Cord Injury. ACS Appl. Mater. Interfaces 2017, 9, 6725–6745. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.T.A.; Kim, Y.M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Darabi, M.; Gu, J.; Shi, J.; Xue, J.; Huang, L.; Liu, Y.; Zhang, L.; Liu, N.; Zhong, W.; et al. A drug delivery hydrogel system based on activin B for Parkinson’s disease. Biomaterials 2016, 102, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Y.; Li, Z.Q.; Li, X.F.; Fan, Z.B.; Liu, Z.G.; Xie, X.Y.; Guan, J.J. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015, 26, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, Z.; Niu, H.; Gao, N.; Guan, Y.; Li, C.; Dang, Y.; Cui, X.; Liu, X.L.; Duan, Y.; et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci. Rep. 2018, 8, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xue, W.J.; Liu, Y.N.; Fan, D.D.; Zhu, C.H.; Ma, X.X. Novel multifunctional PB and PBH hydrogels as soft filler for tissue engineering. J. Mater. Chem. B 2015, 3, 4742–4755. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.F.; Li, X.L.; Li, G.; Tian, T.R.; Lin, S.Y.; Shi, S.R.; Liao, J.F.; Cai, X.X.; Lin, Y.F. Injectable and thermosensitive TGF-beta 1-loaded PCEC hydrogel system for in vivo cartilage repair. Sci. Rep. 2017, 7, 10553. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, Y.; Chen, J.; Chang, F.; Wang, J.; Ding, J.; Chen, X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018, 73, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Kurzhals, S.; Virk, M.; Reimhult, E. Triggered Release from Thermoresponsive Polymersomes with Superparamagnetic Membranes. Materials 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.Y.; Ji, R.; Huang, X.N.; Du, F.S.; Zhang, R.; Liang, D.H.; Li, Z.C. Polymersomes from Dual Responsive Block Copolymers: Drug Encapsulation by Heating and Acid-Triggered Release. Biomacromolecules 2013, 14, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Shanei, A.; Sazgarnia, A.; Dolat, E.; Hojaji-Najafabadi, L.; Sehhati, M.; Baradaran-Ghahfarokhi, M. Dual Function of Gold Nanoparticles in Synergism with Mitoxantrone and Microwave Hyperthermia Against Melanoma Cells. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Hornowski, T.; Kubovcikova, M.; Timko, M.; Koralewski, M.; Jozefczak, A. Heating Induced by Therapeutic Ultrasound in the Presence of Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 11554–11564. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, D.; Sk, U.H.; Sakamoto, Y.; Nakase, I.; Kojima, C. Dual stimuli-sensitive dendrimers: Photothermogenic gold nanoparticle-loaded thermo-responsive elastin-mimetic dendrimers. Colloids Surf. B Biointerfaces 2015, 132, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.F.; Chen, S.W.; Wang, H.M.; Hsieh, S.L.; Wu, C.H.; Chou, H.H.; Hsieh, S.C. Role of Neel and Brownian Relaxation Mechanisms for Water-Based Fe3O4 Nanoparticle Ferrofluids in Hyperthermia. Biomed. Eng.-Appl. Basis C 2010, 22, 393–399. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Rinaldi, C. Thermal potentiation of chemotherapy by magnetic nanoparticles. Nanomedicine 2013, 8, 1689–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossatz, S.; Ludwig, R.; Dahring, H.; Ettelt, V.; Rimkus, G.; Marciello, M.; Salas, G.; Patel, V.; Teran, F.J.; Hilger, I. High Therapeutic Efficiency of Magnetic Hyperthermia in Xenograft Models Achieved with Moderate Temperature Dosages in the Tumor Area. Pharm. Res.-Dordr. 2014, 31, 3274–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dahring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. BCR 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.H.; Wainerdi, S.M.; Cherukuri, T.K.; Kittrell, C.; Wiley, B.J.; Nicholas, N.W.; Curley, S.A.; Kanzius, J.S.; Cherukuri, P. Size-Dependent Joule Heating of Gold Nanoparticles Using Capacitively Coupled Radiofrequency Fields. Nano Res. 2009, 2, 400–405. [Google Scholar] [CrossRef]
- Amini, S.M.; Kharrazi, S.; Rezayat, S.M.; Gilani, K. Radiofrequency electric field hyperthermia with gold nanostructures: Role of particle shape and surface chemistry. Artif. Cells Nanomed. B 2018, 46, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Sharma, S.; Koul, V.; Singh, N. Core-Shell Nanoparticles as an Efficient, Sustained, and Triggered Drug-Delivery System. ACS Omega 2017, 2, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Sivaram, A.J.; Retnakumari, A.P.; Chandran, P.; Malarvizhi, G.L.; Nair, S.; Koyakutty, M. Radiofrequency Ablation of Drug-Resistant Cancer Cells Using Molecularly Targeted Carboxyl-Functionalized Biodegradable Graphene. Adv. Healthc. Mater. 2015, 4, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Paul, A.; Narasimhan, A.; Das, S.K. Analytical prediction of sub-surface thermal history in translucent tissue phantoms during plasmonic photo-thermotherapy (PPTT). J. Therm. Biol. 2016, 62, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Hashida, Y.; Tanaka, H.; Zhou, S.; Kawakami, S.; Yamashita, F.; Murakami, T.; Umeyama, T.; Imahori, H.; Hashida, M. Photothermal ablation of tumor cells using a single-walled carbon nanotube-peptide composite. J. Control. Release 2014, 173, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi-Moghaddam, F.; Azarpira, N.; Sattarahmady, N. Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: An in vitro and animal model investigation. Lasers Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Morales-Dalmau, J.; Vilches, C.; de Miguel, I.; Sanz, V.; Quidant, R. Optimum morphology of gold nanorods for light-induced hyperthermia. Nanoscale 2018, 10, 2632–2638. [Google Scholar] [CrossRef] [PubMed]
- Herynek, V.; Turnovcova, K.; Veverka, P.; Dedourkova, T.; Zvatora, P.; Jendelova, P.; Galisova, A.; Kosinova, L.; Jirakova, K.; Sykova, E. Using ferromagnetic nanoparticles with low Curie temperature for magnetic resonance imaging-guided thermoablation. Int. J. Nanomed. 2016, 11, 3801–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.; Ding, B.; Zhang, S.; Qi, X.; Wang, K.; Tian, J.; Yan, Y.; Ge, Y.; Wu, L. Near-infrared light-responsive nanoparticles with thermosensitive yolk-shell structure for multimodal imaging and chemo-photothermal therapy of tumor. Nanomedicine 2017, 13, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Chen, Y.; Zhou, X.; Chen, D.; Li, Y.; Zhu, X. Hyaluronic Acid-Methotrexate Conjugates Coated Magnetic Polydopamine Nanoparticles for Multimodal Imaging-Guided Multistage Targeted Chemo-Photothermal Therapy. Mol. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Zhang, Y.; Ma, J.Y.; Li, Q.; Li, Y.; Zhou, X.Y.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, G.; Kim, T.H.; Kwon, S.J.; Kim, J.U.; Koh, K.; Park, B.; Hong, H.; Yu, K.J.; Chae, H.; et al. On-Demand Drug Release from Gold Nanoturf for a Thermo- and Chemotherapeutic Esophageal Stent. ACS Nano 2018, 12, 6756–6766. [Google Scholar] [CrossRef] [PubMed]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; LaViolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, X.; He, X.; Wang, K.; Cui, W.; He, D.; Li, D.; Jia, X. Au@Ag/Au nanoparticles assembled with activatable aptamer probes as smart “nano-doctors” for image-guided cancer thermotherapy. Nanoscale 2014, 6, 8754–8761. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, B.; Wang, Y.; Xie, R.; Li, C.; Dou, K.; Gong, S. CuS-Based Theranostic Micelles for NIR-Controlled Combination Chemotherapy and Photothermal Therapy and Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2017, 9, 41700–41711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centelles, M.N.; Wright, M.; So, P.W.; Amrahli, M.; Xu, X.Y.; Stebbing, J.; Miller, A.D.; Gedroyc, W.; Thanou, M. Image-guided thermosensitive liposomes for focused ultrasound drug delivery: Using NIRF-labelled lipids and topotecan to visualise the effects of hyperthermia in tumours. J. Control. Release 2018, 280, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Knights-Mitchell, S.S.; Romanowski, M. Near-Infrared Activated Release of Doxorubicin from Plasmon Resonant Liposomes. Nanotheranostics 2018, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chambre, L.; Degirmenci, A.; Sanyal, R.; Sanyal, A. Multi-Functional Nanogels as Theranostic Platforms: Exploiting Reversible and Nonreversible Linkages for Targeting, Imaging, and Drug Delivery. Bioconjug. Chem. 2018, 29, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Wang, J.; Li, Y.; Han, Z.; Peng, Y.; Zhang, J.; Gao, Z.; Gu, Y.; Deng, D. Quantum Dots-Based Multi-Functional Nano-Prodrug Fabricated by Ingenious Self-Assembly Strategies for Tumor Theranostic. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Lu, N.; Huang, P.; Fan, W.; Wang, Z.; Liu, Y.; Wang, S.; Zhang, G.; Hu, J.; Liu, W.; Niu, G.; et al. Tri-stimuli-responsive biodegradable theranostics for mild hyperthermia enhanced chemotherapy. Biomaterials 2017, 126, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.I.; Cheon, Y.A.; Chung, B.G. Graphene and thermo-responsive polymeric nanocomposites for therapeutic applications. Biomed. Eng. Lett. 2016, 6, 10–15. [Google Scholar] [CrossRef]
- Wen, L.; Yang, S.; Zhong, J.; Zhou, Q.; Xing, D. Thermoacoustic Imaging and Therapy Guidance based on Ultra-short Pulsed Microwave Pumped Thermoelastic Effect Induced with Superparamagnetic Iron Oxide Nanoparticles. Theranostics 2017, 7, 1976–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 131–141. [Google Scholar] [CrossRef] [PubMed]








| Thermo-Sensitive Polymers | Characteristics | Some Applications |
|---|---|---|
| Poly(ethylene glycol)-poly(3-caprolactone)-poly(ethylene glycol) (PCEC) | High gel strength, slow degradation rate and availability in powder form | Sustained release of bevacizumab in glaucoma filtering surgery. Release of paclitaxel for treatment of cervical cancer |
| Poly n-isopropylacrylamide/polyacrylic acid (PNIPAm/PAA) | Display tunable properties. In slightly acidic conditions, the LCST decreases with increase in PAA content | Variety of molecular switching and drug delivery applications where responses to small pH changes are relevant |
| Poly(d,l-lactide)-block poly(ethylene glycol)-block-poly(d,l-lactide) (PDLLA-PEG-PDLLA) | Ability to increase the solubility of hydrophobic compounds, extended release of payloads, biodegradability, excellent safety profile | Multidrug (paclitaxel, rapamycin and 17-AAG heat shock protein inhibitor) release for treatment of ovarian cancer. In combination with black phosphorous as a photothermal platform for postsurgical treatment of cancer. Release of growth factors for skin wound healing |
| Poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) | Improvement in solubility, stability, release and bioavailability of drugs | Sustained release of drugs for vaginal application. Oral drug delivery. Release of nitric oxide for accelerating wound healing |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Moreno, P.; De Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 935. https://doi.org/10.3390/nano8110935
Sánchez-Moreno P, De Vicente J, Nardecchia S, Marchal JA, Boulaiz H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials. 2018; 8(11):935. https://doi.org/10.3390/nano8110935
Chicago/Turabian StyleSánchez-Moreno, Paola, Juan De Vicente, Stefania Nardecchia, Juan A. Marchal, and Houria Boulaiz. 2018. "Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications" Nanomaterials 8, no. 11: 935. https://doi.org/10.3390/nano8110935
APA StyleSánchez-Moreno, P., De Vicente, J., Nardecchia, S., Marchal, J. A., & Boulaiz, H. (2018). Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials, 8(11), 935. https://doi.org/10.3390/nano8110935