In-Situ Preparation of CdTe Quantum Dots Capped with a β-Cyclodextrin-Epichlorohydrin Polymer: Polymer Influence on the Nanocrystal’s Optical Properties
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals
2.2. Preparation of Telluride Precursor (NaHTe) and β-Cyclodextrin Polymer Capped Cadmium Telluride Quantum Dots (CdTe@MPA@βCDP)
2.3. Spectroscopy
2.4. Quantum Yield Measurements
2.5. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. Synthesis and Optical Properties of βCDP Capped QDs
3.1.1. Effects on the First Exciton and Fluorescence Spectra
3.1.2. Stokes-Shift and Full Width at Half Maximum (FWHM) of the Emission Band
3.2. ATR-FTIR Characterization of the In-Situ Prepared CdTe@MPA QDs Capped with βCDP
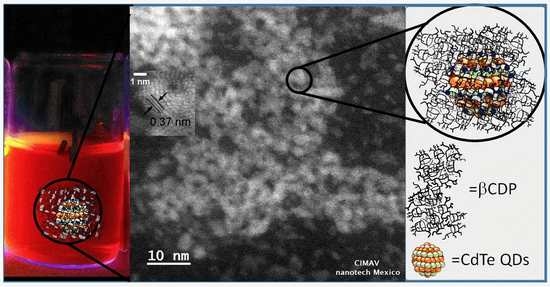
3.3. High Resolution Transmission Electron Microscopy Characterization (HRTEM)
- (i)
- The presence of the βCDP on the CdTe QDs surface brings in an additional diffusion step to the precursors (Cd-MPA and HTe-) within the polymer network before reaching the QDs surface.
- (ii)
- The presence of the βCDP attached to MPA, on the QDs surface, alters the electrostatic nature of the QDs surface. These situations are described in Scheme 2.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klimov, V.I. Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0203913264. [Google Scholar]
- Jing, L.; Kershaw, S.V.; Li, Y.; Huang, X.; Li, Y.; Rogach, A.L.; Gao, M. Aqueous Based Semiconductor Nanocrystals. Chem. Rev. 2016, 116, 10623–10730. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A. Small-Particle Research: Physicochemical Properties of Extremely Small Colloidal Metal and Semiconductor Particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Fendler, J.H. Atomic and Molecular Clusters in Membrane Mimetic Chemistry. Chem. Rev. 1987, 87, 877–899. [Google Scholar] [CrossRef]
- Vossmeyer, T.; Reck, G.; Katsikas, L.; Haupt, E.T.K.; Schulz, B.; Weller, H. A “double-diamond superlattice” built up of Cd17S4(SCH2CH2OH)26 clusters. Science 1995, 267, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef] [PubMed]
- Lesnyak, V.; Gaponik, N.; Eychmüller, A. Colloidal semiconductor nanocrystals: The aqueous approach. Chem. Soc. Rev. 2013, 42, 2905–2929. [Google Scholar] [CrossRef] [PubMed]
- Gaponik, N.; Talapin, D.V.; Rogach, A.L.; Hoppe, K.; Shevchenko, E.V.; Kornowski, A.; Eychmüller, A.; Weller, H. Thiol-capping of CDTe nanocrystals: An alternative to organometallic synthetic routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Gaponik, N.; Rogach, A.L. Thiol-capped CdTe nanocrystals: Progress and perspectives of the related research fields. Phys. Chem. Chem. Phys. 2010, 12, 8685–8693. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Duan, H.; Mohs, A.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Tenhu, H. Recent advances in polymer protected gold nanoparticles: Synthesis, properties and applications. Chem. Commun. (Camb.) 2007, 4580–4598. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular Chemistry of Cyclodextrins in Enzyme Technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Puskás, I.; Szemjonov, A.; Fenyvesi, É.; Malanga, M.; Szente, L. Aspects of determining the molecular weight of cyclodextrin polymers and oligomers by static light scattering. Carbohydr. Polym. 2013, 94, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Sánchez, I.; Cao, R.; Rieumont, J. Solubility and Kinetic Release Studies of Naproxen and Ibuprofen in Soluble Epichlorohydrin-β-cyclodextrin Polymer. Supramol. Chem. 2006, 18, 627–631. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Sedghamiz, T. Chiral recognition of Propranolol enantiomers by β-Cyclodextrin: Quantum chemical calculation and molecular dynamics simulation studies. Chem. Phys. 2014, 445, 5–13. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Zhang, C. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Martin-Trasanco, R.; Cao, R.; Esparza-Ponce, H.E.; García-Pupo, L.; Montero-Cabrera, M.E. Small, stable and biocompatible gold nanoparticles capped with a β-cyclodextrin polymer. RSC Adv. 2015, 5, 98440–98446. [Google Scholar] [CrossRef]
- Renard, E.; Deratani, A.; Volet, G.; Sebille, B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997, 33, 49–57. [Google Scholar] [CrossRef]
- Martin-Trasanco, R.; Cao, R.; Esparza-Ponce, H.E.; Montero-Cabrera, M.E.; Arratia-Pérez, R. Reduction of Au(III) by a β-cyclodextrin polymer in acid medium. A stated unattainable reaction. Carbohydr. Polym. 2017, 175, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Z.; Yang, B.; Gao, M. The Influence of Carboxyl Groups on the Photoluminescence of Mercaptocarboxylic Acid-Stabilized CdTe Nanoparticles. J. Phys. Chem. B 2003, 107, 8–13. [Google Scholar] [CrossRef]
- Grabolle, M.; Spieles, M.; Lesnyak, V.; Gaponik, N.; Eychmüller, A.; Resch-Genger, U. Determination of the Fluorescence Quantum Yield of Quantum Dots: Suitable Procedures and Achievable Uncertainties. Anal. Chem. 2009, 81, 6285–6294. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogach, A.L.; Franzl, T.; Klar, T.A.; Feldmann, J.; Gaponik, N.; Lesnyak, V.; Shavel, A.; Eychmüller, A.; Rakovich, Y.P.; Donegan, J.F. Aqueous Synthesis of Thiol-Capped CdTe Nanocrystals: State-of-the-Art. J. Phys. Chem. C 2007, 111, 14628–14637. [Google Scholar] [CrossRef]
- Baskoutas, S.; Terzis, A.F. Size dependent exciton energy of various technologically important colloidal quantum dots. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2008, 147, 280–283. [Google Scholar] [CrossRef]
- Baskoutas, S.; Terzis, A.F. Size-dependent band gap of colloidal quantum dots. J. Appl. Phys. 2006, 99, 013708. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental Determination of the Extinction Coefficient of CdTe, CdSe, and CdS Nanocrystals. Chem. Mater. 2003, 125, 2854–2860. [Google Scholar] [CrossRef]
- Nirmal, M.; Brus, L. Luminescence Photophysics in Semiconductor Nanocrystals. Acc. Chem. Res. 1999, 32, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Beane, G.; Kelley, D.F. Strain release in metastable CdSe/CdS quantum dots. Chem. Phys. 2016, 471, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Egyed, O. Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1990, 1, 225–227. [Google Scholar] [CrossRef]
- Li, L.; Qian, H.; Fang, N.; Ren, J. Significant enhancement of the quantum yield of CdTe nanocrystals synthesized in aqueous phase by controlling the pH and concentrations of precursor solutions. J. Lumin. 2006, 116, 59–66. [Google Scholar] [CrossRef]
- Rogach, A.L. Nanocrystalline CdTe and CdTe(S) particles: Wet chemical preparation, size-dependent optical properties and perspectives of optoelectronic applications. Mater. Sci. Eng. B 2000, 69–70, 435–440. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, C.; Zhang, J.; Sun, H.; Li, M.; Yang, B. Directing the Growth of Semiconductor Nanocrystals in Aqueous Solution: Role of Electrostatics. ChemPhysChem 2008, 9, 1309–1316. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Trasanco, R.; Esparza-Ponce, H.E.; Ortiz, P.D.; Oyarzun, D.P.; Zuñiga, C.; Montero-Cabrera, M.E.; Tundidor-Camba, A.; Pizarro, G.d.C.; Arratia-Pérez, R. In-Situ Preparation of CdTe Quantum Dots Capped with a β-Cyclodextrin-Epichlorohydrin Polymer: Polymer Influence on the Nanocrystal’s Optical Properties. Nanomaterials 2018, 8, 948. https://doi.org/10.3390/nano8110948
Martin-Trasanco R, Esparza-Ponce HE, Ortiz PD, Oyarzun DP, Zuñiga C, Montero-Cabrera ME, Tundidor-Camba A, Pizarro GdC, Arratia-Pérez R. In-Situ Preparation of CdTe Quantum Dots Capped with a β-Cyclodextrin-Epichlorohydrin Polymer: Polymer Influence on the Nanocrystal’s Optical Properties. Nanomaterials. 2018; 8(11):948. https://doi.org/10.3390/nano8110948
Chicago/Turabian StyleMartin-Trasanco, Rudy, Hilda E. Esparza-Ponce, Pedro D. Ortiz, Diego P. Oyarzun, Cesar Zuñiga, Maria E. Montero-Cabrera, Alain Tundidor-Camba, Guadalupe del C. Pizarro, and Ramiro Arratia-Pérez. 2018. "In-Situ Preparation of CdTe Quantum Dots Capped with a β-Cyclodextrin-Epichlorohydrin Polymer: Polymer Influence on the Nanocrystal’s Optical Properties" Nanomaterials 8, no. 11: 948. https://doi.org/10.3390/nano8110948
APA StyleMartin-Trasanco, R., Esparza-Ponce, H. E., Ortiz, P. D., Oyarzun, D. P., Zuñiga, C., Montero-Cabrera, M. E., Tundidor-Camba, A., Pizarro, G. d. C., & Arratia-Pérez, R. (2018). In-Situ Preparation of CdTe Quantum Dots Capped with a β-Cyclodextrin-Epichlorohydrin Polymer: Polymer Influence on the Nanocrystal’s Optical Properties. Nanomaterials, 8(11), 948. https://doi.org/10.3390/nano8110948