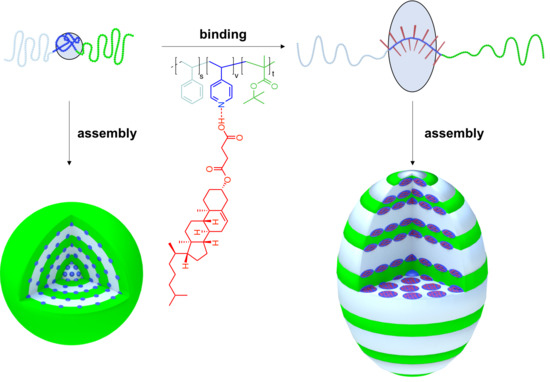
Supramolecular Modification of ABC Triblock Terpolymers in Confinement Assembly
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Shea, K.J.; Kitagawa, S.; Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cellsw. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar]
- Gröschel, A.H.; Müller, A.H.E. Self-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Ruokolainen, J. Switching supramolecular polymeric materials with multiple length scales. Science 1998, 280, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, J.; ten Brinke, G.; Ikkala, O. Supramolecular polymeric materials with hierarchical structure-within-structure morphologies. Adv. Mater. 1999, 11, 777–780. [Google Scholar] [CrossRef]
- Van Zoelen, W.; Asumaa, T.; Ruokolainen, J.; Ikkala, O.; ten Brinke, G. Phase behavior of solvent vapor annealed thin films of PS-b-P4VP(PDP) supramolecules. Macromolecules 2008, 41, 3199–3208. [Google Scholar] [CrossRef]
- Valkama, S.; Kosonen, H.; Ruokolainen, J.; Haatainen, T.; Torkkeli, M.; Serimaa, R.; ten Brinke, G.; Ikkala, O. Self-assembled polymeric solid films with temperature-induced large and reversible photonic-bandgap switching. Nat. Mater. 2004, 3, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukovic, I.; ten Brinke, G.; Loos, K. Hexagonally perforated layer morphology in PS-b-P4VP(PDP) supramolecules. Macromolecules 2012, 45, 9409–9418. [Google Scholar] [CrossRef]
- Hofman, A.H.; Reza, M.; Ruokolainen, J.; ten Brinke, G.; Loos, K. Hierarchical layer engineering using supramolecular double-comb diblock copolymers. Angew. Chem. Int. Ed. 2016, 55, 13081–13085. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Retsch, M.; Fustin, C.A.; Del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wylie, R.A.L.; Klinger, D.; Connal, L.A. Shape control of soft nanoparticles and their assemblies. Chem. Mater. 2017, 29, 1918–1945. [Google Scholar] [CrossRef]
- Yabu, H.; Higuchi, T.; Jinnai, H. Frustrated phases: Polymeric self-assemblies in a 3D confinement. Soft Matter 2014, 10, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Fan, H. Self-assembly of nanostructured block copolymer nanoparticles. Soft Matter 2014, 10, 9212–9219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.P.; Yi, G.-R. Nanostructured colloidal particles by confined self-assembly of block copolymers in evaporative droplets. Front. Mater. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Y.; Jiang, W. Recent progress in the self-assembly of block copolymers confined in emulsion droplet. Chem. Commun. 2018, 54, 13183–13195. [Google Scholar] [CrossRef] [PubMed]
- Liljeström, V.; Chen, C.; Dommersnes, P.; Fossum, J.O.; Gröschel, A.H. Active structuring of colloids through field-driven self-assembly. Curr. Opin. Colloid Interface Sci. 2019, 40, 25–41. [Google Scholar] [CrossRef]
- Kister, T.; Mravlak, M.; Schilling, T.; Kraus, T. Pressure-controlled formation of crystalline, Janus, and core–shell supraparticles. Nanoscale 2016, 8, 13377–13384. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Qiao, Q.; Zhu, Y.; Ouyang, M. Colloidal binary supracrystals with tunable structural lattices. J. Am. Chem. Soc. 2018, 140, 9095–9098. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Shen, X.; Yao, L.; Wang, L.; Chen, X.; Xie, S.; Li, T.; Hu, J.; Yang, D.; et al. Scalable assembly of crystalline binary nanocrystal superparticles and their enhanced magnetic and electrochemical properties. J. Am. Chem. Soc. 2018, 140, 15038–15047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jun-Yan Suen, J.; Prince, E.; Larin, E.M.; Klinkova, A.; Thérien-Aubin, H.; Zhu, S.; Yang, B.; Helmy, A.S.; Lavrentovich, O.D.; et al. Colloidal cholesteric liquid crystal in spherical confinement. Nat. Commun. 2016, 7, 12520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical self-assembly of cellulose nanocrystals in a confined geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- Chen, L.; Yu, S.; Wang, H.; Xu, J.; Liu, C.; Chong, W.H.; Chen, H. General methodology of using oil-in-water and water-in-oil emulsions for coiling nanofilaments. J. Am. Chem. Soc. 2013, 135, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, B.; Jin, Q.; Ding, D.; Shi, A.-C. Confined self-assembly of cylinder-forming diblock copolymers: Effects of confining geometries. Soft Matter 2011, 7, 10227. [Google Scholar] [CrossRef]
- Huh, J.; Park, C.; Kwon, Y.K. Commensurability effect in diblock copolymer lamellar phase under d-dimensional nanoconfinement. J. Chem. Phys. 2010, 133, 114903. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Sloan, C.R.; Thomas, E.L. Interplay of symmetries of block polymers and confining geometries. Eur. Polym. J. 2011, 47, 630–646. [Google Scholar] [CrossRef]
- Shi, A.-C.; Li, B. Self-assembly of diblock copolymers under confinement. Soft Matter 2013, 9, 1398–1413. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Elbert, J.; Scheid, D.; Hawker, C.J.; Klinger, D.; Gallei, M. Metallopolymer-based shape anisotropic nanoparticles. ACS Macro Lett. 2015, 53, 731–735. [Google Scholar] [CrossRef]
- Higuchi, T.; Motoyoshi, K.; Sugimori, H.; Jinnai, H.; Yabu, H.; Shimomura, M. Phase transition and phase transformation in block copolymer nanoparticles. Macromol. Rapid Commun. 2010, 31, 1773–1778. [Google Scholar] [CrossRef]
- Connal, L.A.; Lynd, N.A.; Robb, M.J.; See, K.A.; Jang, S.G.; Spruell, J.M.; Hawker, C.J. Mesostructured block copolymer nanoparticles: Versatile templates for hybrid inorganic/organic nanostructures. Chem. Mater. 2012, 24, 4036–4042. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, M.P.; Yang, H.; Ku, K.H.; Jang, S.G.; Youm, K.H.; Yi, G.R.; Kim, B.J. Monodipserse nanostructured spheres of block copolymers and nanoparticles via cross-flow membrane emulsification. Chem. Mater. 2015, 27, 6314–6321. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Yi, G.; Yang, S. Cooperative assembly of block copolymers with deformable interfaces: Toward nanostructured particles. Adv. Mater. 2008, 20, 4103–4108. [Google Scholar] [CrossRef]
- Li, W.; Wickham, R.A. Self-assembled morphologies of a diblock copolymer melt confined in a cylindrical nanopore. Macromolecules 2006, 39, 8492–8498. [Google Scholar] [CrossRef]
- Grundy, L.S.; Lee, V.E.; Li, N.; Sosa, C.; Mulhearn, W.D.; Liu, R.; Register, R.A.; Nikoubashman, A.; Prud’homme, R.K.; Panagiotopoulos, A.Z.; et al. Rapid production of internally structured colloids by flash nanoprecipitation of block copolymer blends. ACS Nano 2018, 12, 4660–4668. [Google Scholar] [CrossRef]
- Yabu, H.; Jinno, T.; Koike, K.; Higuchi, T.; Shimomura, M. Nanoparticle arrangements in block copolymer particles with microphase-separated structures. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1717–1722. [Google Scholar] [CrossRef]
- Jang, S.G.; Audus, D.J.; Klinger, D.; Krogstad, D.V.; Kim, B.J.; Cameron, A.; Kim, S.; Delaney, K.T.; Hur, S.; Killops, K.L.; et al. Striped, ellipsoidal particles by controlled assembly of diblock copolymers. J. Am. Chem. Soc. 2013, 135, 6649–6657. [Google Scholar] [CrossRef]
- Klinger, D.; Robb, M.J.; Spruell, J.M.; Lynd, N.A.; Hawker, C.J.; Connal, L.A. Supramolecular guests in solvent driven block copolymer assembly: From internally structured nanoparticles to micelles. Polym. Chem. 2013, 4, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Soininen, A.J.; Rahikkala, A.; Korhonen, J.T.; Kauppinen, E.I.; Mezzenga, R.; Raula, J.; Ruokolainen, J. Hierarchical structures of hydrogen-bonded liquid-crystalline side-chain diblock copolymers in nanoparticles. Macromolecules 2012, 45, 8743–8751. [Google Scholar] [CrossRef]
- Deng, R.; Liu, S.; Li, J.; Liao, Y.; Tao, J.; Zhu, J. Mesoporous block copolymer nanoparticles with tailored structures by hydrogen-bonding-assisted self-assembly. Adv. Mater. 2012, 24, 1889–1893. [Google Scholar] [CrossRef]
- Stadler, R.; Auschra, C.; Beckmann, J.; Krappe, U.; Voight-Martin, I.; Leibler, L. Morphology and thermodynamics of symmetric poly(A-block-B-block-C) triblock copolymers. Macromolecules 1995, 28, 3080–3097. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Abetz, V.; Stadler, R. Cylindrical morphologies in asymmetric ABC triblock copolymers. Macromol. Chem. Phys. 1997, 198, 1051–1083. [Google Scholar] [CrossRef]
- Giebeler, E.; Stadler, R. ABC triblock polyampholytes containing a neutral hydrophobic block, a polyacid and a polybase. Macromol. Chem. Phys. 1997, 198, 3815–3825. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Jakob, T.; Abetz, V.; Stadler, R. Spheres on spheres—A novel spherical multiphase morphology in polystyrene-block-polybutadiene-block-poly(methyl methacrylate) triblock copolymers. Polym. Bull. 1998, 40, 219–226. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Mogi, Y.; Nomura, M.; Kotsuji, H.; Ohnishi, K.; Matsushita, Y.; Noda, I. Superlattice structures in morphologies of the ABC triblock copolymers. Macromolecules 1994, 27, 6755–6760. [Google Scholar] [CrossRef]
- Epps, T.H.; Cochran, E.W.; Bailey, T.S.; Waletzko, R.S.; Hardy, C.M.; Bates, F.S. Ordered network phases in linear poly(isoprene-b-styrene-b-ethylene oxide) triblock copolymers. Macromolecules 2004, 37, 8325–8341. [Google Scholar] [CrossRef]
- Löbling, T.I.; Hiekkataipale, P.; Hanisch, A.; Bennet, F.; Schmalz, H.; Ikkala, O.; Gröschel, A.H.; Müller, A.H.E. Bulk morphologies of polystyrene-block-polybutadiene-block-poly(tert-butyl methacrylate) triblock terpolymers. Polymer 2015, 72, 479–489. [Google Scholar] [CrossRef]
- Gobius du Sart, G.; Rachmawati, R.; Voet, V.; Alberda van Ekenstein, G.; Polushkin, E.; ten Brinke, G.; Loos, K. Poly(tert-butyl methacrylate-b-styrene-b-4-vinylpyridine) triblock copolymers: Synthesis, interactions, and self-assembly. Macromolecules 2008, 41, 6393–6399. [Google Scholar] [CrossRef]
- Gobius du Sart, G.; Vukovic, I.; Alberda van Ekenstein, G.; Polushkin, E.; Loos, K.; ten Brinke, G. Self-assembly of supramolecular triblock copolymer complexes. Macromolecules 2010, 43, 2970–2980. [Google Scholar] [CrossRef]
- Du Sart, G.G.; Vukovic, I.; Vukovic, Z.; Polushkin, E.; Hiekkataipale, P.; Ruokolainen, J.; Loos, K.; ten Brinke, G. Nanoporous network channels from self-assembled triblock copolymer supramolecules. Macromol. Rapid Commun. 2011, 32, 366–370. [Google Scholar] [CrossRef]
- Jiang, S.; Göpfert, A.; Abetz, V. Novel morphologies of block copolymer blends via hydrogen bonding. Macromolecules 2003, 36, 6171–6177. [Google Scholar] [CrossRef]
- Hiekkataipale, P.; Löbling, T.I.; Poutanen, M.; Priimagi, A.; Abetz, V.; Ikkala, O.; Gröschel, A.H. Controlling the shape of Janus nanostructures through supramolecular modification of ABC terpolymer bulk morphologies. Polymer 2016, 107, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Hofman, A.H.; Terzic, I.; Stuart, M.C.A.; Ten Brinke, G.; Loos, K. Hierarchical self-assembly of supramolecular double-comb triblock terpolymers. ACS Macro Lett. 2018, 7, 1168–1173. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Chen, Y.; Yang, Z. Onion-like microspheres with tricomponent from gelable triblock copolymers. J. Colloid Interface Sci. 2010, 346, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Li, J.; Zhou, H.; Xie, X.; Zhu, J. ABC triblock copolymer particles with tunable shape and internal structure through 3D confined assembly. Macromolecules 2015, 48, 2628–2636. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Wang, K.; Li, J.; Zhou, H.; Xie, X.; Zhu, J. Additives induced structural transformation of ABC triblock copolymer particles. Langmuir 2015, 31, 10975–10982. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Liang, F.; Li, W.; Liu, S.; Liang, R.; Cai, M.; Yang, Z.; Zhu, J. Shaping functional nano-objects by 3D confined supramolecular assembly. Small 2013, 9, 4099–4103. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.; Xu, J.; Hwang, M.-S.; Tian, D.; Wang, K.; Zhang, L.; Liao, Y.; Park, H.-G.; Yi, G.-R.; et al. Responsive block copolymer photonic microspheres. Adv. Mater. 2018, 30, 1707344. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Y.; Jiang, W. Self-assembly of ABC triblock copolymers under 3D soft confinement: A Monte Carlo study. Soft Matter 2016, 12, 965–972. [Google Scholar] [CrossRef]
- Saccone, M.; Spengler, M.; Pfletscher, M.; Kunze, K.; Virkki, M.; Wölper, C.; Gehrke, R.; Metrangolo, P.; Priimagi, A.; Giese, M. Photoresponsive halogen-bonded liquid crystals: The role of aromatic fluorine substitution. Chem. Mater. 2018. accepted. [Google Scholar]
- Cesteros, L.C.; Isasi, J.R.; Katime, I. Hydrogen bonding in poly(4-vinylpyridine)/poly(vinyl acetate-co-vinyl alcohol) blends. An infrared study. Macromolecules 1993, 26, 7256–7262. [Google Scholar] [CrossRef]
- Priimagi, A.; Saccone, M.; Cavallo, G.; Shishido, A.; Pilati, T.; Metrangolo, P.; Resnati, G. Photoalignment and surface-relief-grating formation are efficiently combined in low-molecular-weight halogen-bonded complexes. Adv. Mater. 2012, 24, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, A.; Pelras, T.; Chakroun, R.; Gröschel, A.H.; Müllner, M. Self-assembly of diblock molecular polymer brushes in the spherical confinement of nanoemulsion droplets. Macromol. Rapid Commun. 2018, 39, e1800177. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Wang, C.X.; Connal, L.A.; Audus, D.J.; Jang, S.G.; Kraemer, S.; Killops, K.L.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. A facile synthesis of dynamic, shape-changing polymer particles. Angew. Chem. Int. Ed. 2014, 53, 7018–7022. [Google Scholar] [CrossRef] [PubMed]







| Code | Mn in [kg/mol] a | PS | PV | PT | fS in [wt %] | fV in [wt %] | fT in [wt %] |
|---|---|---|---|---|---|---|---|
| SVT1 | 195 | 769 | 315 | 577 | 41 | 17 | 42 |
| SVT2 | 59 | 210 | 67 | 212 | 37 | 12 | 54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintieri, G.; Saccone, M.; Spengler, M.; Giese, M.; Gröschel, A.H. Supramolecular Modification of ABC Triblock Terpolymers in Confinement Assembly. Nanomaterials 2018, 8, 1029. https://doi.org/10.3390/nano8121029
Quintieri G, Saccone M, Spengler M, Giese M, Gröschel AH. Supramolecular Modification of ABC Triblock Terpolymers in Confinement Assembly. Nanomaterials. 2018; 8(12):1029. https://doi.org/10.3390/nano8121029
Chicago/Turabian StyleQuintieri, Giada, Marco Saccone, Matthias Spengler, Michael Giese, and André H. Gröschel. 2018. "Supramolecular Modification of ABC Triblock Terpolymers in Confinement Assembly" Nanomaterials 8, no. 12: 1029. https://doi.org/10.3390/nano8121029
APA StyleQuintieri, G., Saccone, M., Spengler, M., Giese, M., & Gröschel, A. H. (2018). Supramolecular Modification of ABC Triblock Terpolymers in Confinement Assembly. Nanomaterials, 8(12), 1029. https://doi.org/10.3390/nano8121029