Hexosomes with Undecylenic Acid Efficient against Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Hexosomes
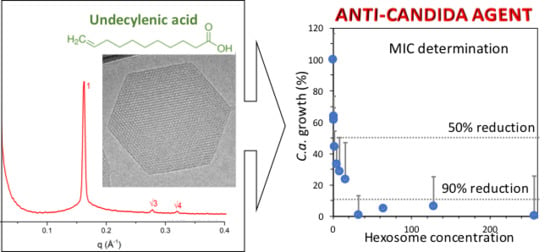
2.2. Anti-Candida Properties of Hexosomes
3. Materials and Methods
3.1. Formulation of Hexosomes
3.2. Physicochemical Characterisation of Hexosomes
3.3. Microorganism and Culture Conditions
3.4. Anti-Candida Characterization of Hexosomes
3.4.1. Minimum Inhibitory Concentration (MIC)
3.4.2. XTT Assay
3.4.3. Embedded Filamentation Assay
3.5. Cytotoxicity Study of Hexosomes
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Hube, B. Candida albicans morphology: Still in focus. Expert Rev. Anti-Infect. Ther. 2017, 15, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Romo, J.A.; Pierce, C.G.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Targeting Candida albicans filamentation for antifungal drug development. Virulence 2017, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Otlewska, A.; Kunicka-Styczyńska, A.; Krajewska, A. Candida albicans Impairments Induced by Peppermint and Clove Oils at Sub-Inhibitory Concentrations. Int. J. Mol. Sci. 2017, 18, 1307. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Skupien, J.A.; Valentini, F.; Boscato, N.; Pereira-Cenci, T. Prevention and treatment of Candida colonization on denture liners: A systematic review. J. Prosthet. Dent. 2013, 110, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Milošević, I.; Guillot, S.; Tadić, M.; Duttine, M.; Duguet, E.; Pierzchala, K.; Sienkiewicz, A.; Forró, L.; Saboungi, M.-L. Loading and release of internally self-assembled emulsions embedded in a magnetic hydrogel. Appl. Phys. Lett. 2014, 104, 043701. [Google Scholar] [CrossRef]
- Serieye, S.; Méducin, F.; Milošević, I.; Fu, L.; Guillot, S. Interface tuning and stabilization of monoglyceride mesophase dispersions: Food emulsifiers and mixtures efficiency. J. Colloid Interface Sci. 2017, 496, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Moitzi, C.; Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Direct and indirect thermal transitions from hexosomes to emulsified micro-emulsions in oil-loaded monoglyceride-based particles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 291, 78–84. [Google Scholar] [CrossRef]
- Salonen, A.; Guillot, S.; Glatter, O. Determination of Water Content in Internally Self-Assembled Monoglyceride-Based Dispersions from the Bulk Phase. Langmuir 2007, 23, 9151–9154. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Venugopal, E.; Ramagiri, S.; Bellare, J.R.; Kumaraswamy, G.; Singh, N. Enhancing Cubosome Functionality by Coating with a Single Layer of Poly-ε-lysine. ACS Appl. Mater. Interfaces 2014, 6, 17126–17133. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Barrios-Lopez, B.; Kallinen, A.; Laurinmäki, P.; Butcher, S.J.; Raki, M.; Weisell, J.; Bergström, K.; Larsen, S.W.; Østergaard, J.; et al. SPECT/CT imaging of radiolabeled cubosomes and hexosomes for potential theranostic applications. Biomaterials 2013, 34, 8491–8503. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Kyriakos, K.; Schneider, F.; Dietz, H.; Winter, G.; Papadakis, C.M.; Hubert, M. Characterization of Lipid-Based Hexosomes as Versatile Vaccine Carriers. Mol. Pharm. 2016, 13, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Hexosomes: A Novel Drug Delivery System|BenthamScience. Available online: http://www.eurekaselect.com/70915/article (accessed on 11 December 2017).
- Dong, Y.-D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and Dispersed Aqueous Phase Behavior of Phytantriol: Effect of Vitamin E Acetate and F127 Polymer on Liquid Crystal Nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Moitzi, C.; Guillot, S.; Fritz, G.; Salentinig, S.; Glatter, O. Phase Reorganization in Self-Assembled Systems through Interparticle Material Transfer. Adv. Mater. 2007, 19, 1352–1358. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. Available online: https://www.hindawi.com/journals/bmri/2014/815981/ (accessed on 29 August 2017).
- Du, J.D.; Liu, Q.; Salentinig, S.; Nguyen, T.-H.; Boyd, B.J. A novel approach to enhance the mucoadhesion of lipid drug nanocarriers for improved drug delivery to the buccal mucosa. Int. J. Pharm. 2014, 471, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, N.K.; Jain, V.; Dubey, V.; Mishra, D.; Jain, N.K. Enhanced Oromucosal Delivery of Progesterone via Hexosomes. Pharm. Res. 2007, 24, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.L.; Rothman, S. Undecylenic acid in the treatment of dermatomycosis. Arch. Dermatol. Syphilol. 1945, 52, 166–171. [Google Scholar] [CrossRef]
- Shi, D.; Zhao, Y.; Yan, H.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Qiu, Y.; Li, D.; Liu, W. Antifungal effects of undecylenic acid on the biofilm formation of Candida albicans. Int. J. Clin. Pharmacol. Ther. 2016, 54, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wyss, O.; Ludwig, B.J.; Joiner, R.R. The fungistatic and fungicidal action of fatty acids and related compounds. Arch. Biochem. 1945, 7, 415–425. [Google Scholar]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Chapter Five-Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes). In Advances in Planar Lipid Bilayers and Liposomes; Iglič, A., Kulkarni, C.V., Rappolt, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 21, pp. 131–187. [Google Scholar]
- Sun, X.; Lin, W.; Li, X.; Shen, Q.; Luo, H. Detection and quantification of extra virgin olive oil adulteration with edible oils by FT-IR spectroscopy and chemometrics. Anal. Methods 2015, 7, 3939–3945. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Control of the Internal Structure of MLO-Based Isasomes by the Addition of Diglycerol Monooleate and Soybean Phosphatidylcholine. Langmuir 2006, 22, 9919–9927. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Salentinig, S.; Chemelli, A.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Influence of the Stabilizer Concentration on the Internal Liquid Crystalline Order and the Size of Oil-Loaded Monolinolein-Based Dispersions. Langmuir 2010, 26, 6222–6229. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.T. Bicontinuous structures in lyotropic liquid crystals and crystalline hyperbolic surfaces. Curr. Opin. Solid State Mater. Sci. 1996, 1, 653–662. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Del Bel Cury, A.A.; Sartoratto, A.; Garcia Rehder, V.L.; Silva, W.J. Effects of Undecylenic Acid Released from Denture Liner on Candida Biofilms. J. Dent. Res. 2012, 91, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Bonvin, D.; Hofmann, H.; Mionić Ebersold, M. Fungicidal PMMA-undecylenic acid composites. Int. J. Mol. Sci. 2018, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Mekala, S.; Chaffin, W.L. Role for Cell Density in Antifungal Drug Resistance in Candida albicans Biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Cell Density and Cell Aging as Factors Modulating Antifungal Resistance of Candida albicans Biofilms. Antimicrob. Agents Chemother. 2008, 52, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; de Koster, C.G.; Brul, S. Cell Wall-Related Bionumbers and Bioestimates of Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell 2014, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.K.; Deveau, A.; Piispanen, A.E.; Hogan, D.A. Farnesol and Cyclic AMP Signaling Effects on the Hypha-to-Yeast Transition in Candida albicans. Eukaryot. Cell 2012, 11, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Pendrak, M.L.; Roberts, D.D. Hbr1 Activates and Represses Hyphal Growth in Candida albicans and Regulates Fungal Morphogenesis under Embedded Conditions. PLoS ONE 2015, 10, e0126919. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, D.; Hofmann, H.; Ebersold, M.M. Assessment of nanoparticles’ safety: Corrected absorbance-based toxicity test. Analyst 2017, 142, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Danino, D.; Raghavan, S.R. Polymerizable Vesicles Based on a Single-Tailed Fatty Acid Surfactant: A Simple Route to Robust Nanocontainers. Langmuir 2009, 25, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Nyame Mendendy Boussambe, G.; Valentin, R.; Fabre, J.-F.; Navailles, L.; Nallet, F.; Gaillard, C.; Mouloungui, Z. Self-Assembling Behavior of Glycerol Monoundecenoate in Water. Langmuir 2017, 33, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Bulut, F.; Yulug, N.; Yalabik-Kaş, H.S.; Hincal, A.A. Antifungal Activity of Undecylenic Acid Emulsions by Microbiological Methods. Drug Dev. Ind. Pharm. 1982, 8, 847–856. [Google Scholar] [CrossRef]
- McLain, N.; Ascanio, R.; Baker, C.; Strohaver, R.A.; Dolan, J.W. Undecylenic Acid Inhibits Morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Deng, D.M.; Kraneveld, E.A.; Manders, E.M.M.; Del Bel Cury, A.A.; ten Cate, J.M.; Crielaard, W. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 2008, 53, 755–764. [Google Scholar] [CrossRef] [PubMed]
- COE-SOFT-Soft Denture Reline Material. Available online: http://www.gcamerica.com/products/operatory/COE-Soft/ (accessed on 26 November 2017).






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mionić Ebersold, M.; Petrović, M.; Fong, W.-K.; Bonvin, D.; Hofmann, H.; Milošević, I. Hexosomes with Undecylenic Acid Efficient against Candida albicans. Nanomaterials 2018, 8, 91. https://doi.org/10.3390/nano8020091
Mionić Ebersold M, Petrović M, Fong W-K, Bonvin D, Hofmann H, Milošević I. Hexosomes with Undecylenic Acid Efficient against Candida albicans. Nanomaterials. 2018; 8(2):91. https://doi.org/10.3390/nano8020091
Chicago/Turabian StyleMionić Ebersold, Marijana, Milica Petrović, Wye-Khay Fong, Debora Bonvin, Heinrich Hofmann, and Irena Milošević. 2018. "Hexosomes with Undecylenic Acid Efficient against Candida albicans" Nanomaterials 8, no. 2: 91. https://doi.org/10.3390/nano8020091
APA StyleMionić Ebersold, M., Petrović, M., Fong, W. -K., Bonvin, D., Hofmann, H., & Milošević, I. (2018). Hexosomes with Undecylenic Acid Efficient against Candida albicans. Nanomaterials, 8(2), 91. https://doi.org/10.3390/nano8020091