Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
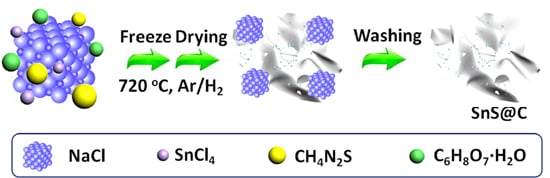
2.1. Synthesis of SnS Decorated Carbon Nano-Networks
2.2. Materials Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Qi, T.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Wan, L.; Yang, S.H.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bo, S.; Cui, W.; Li, F.; Wang, C.; Xia, Y. Nano-sized cobalt oxide/mesoporous carbon sphere composites as negative electrode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 6497–6503. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 nanobelt@Co9S8 composites as promising anode materials for lithium and sodium ion batteries. Nanomaterials 2017, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.D., II; Hentz, O.D.; Chen, S.; Wang, D.; Schaak, R.E. Formation of SnS nanoflowers for lithium ion batteries. Chem. Commun. 2012, 48, 5608–5610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Feng, J.; Jiang, X.; Yue, J.; Yang, J.; Qian, Y. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Ma, Q.; Zhuang, Q.; Liang, J.; Zhang, Z.; Liu, J.; Peng, H.; Mao, C.; Li, G. Novel mesoporous flowerlike iron sulfide hierarchitectures: Facile synthesis and fast lithium storage capability. Nanomaterials 2017, 7, 431. [Google Scholar] [CrossRef]
- Cai, J.; Li, Z.; Shen, P. Porous SnS nanorods/carbon hybrid materials as highly stable and high capacity anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4093–4098. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhu, J.; Rui, X.; Cao, X.; Chen, C.; Zhang, H.; Hng, H.H.; Yan, Q. Controlled synthesis of carbon-coated cobalt sulfide nanostructures in oil phase with enhanced Li storage performances. ACS Appl. Mater. Interfaces 2012, 4, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Liu, S.; Yang, J.; Qian, Y. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: A high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 2015, 7, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiu, Z.; Li, D.; Ullah, S.; Hai, Y.; Xin, H.; Liao, W.; Yang, B.; Fan, H.; Xu, J.; Zhu, C. NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries. Energy Storage Mater. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Wei, X.; Li, W.; Shi, J.; Gu, L.; Yu, Y. FeS@C on carbon cloth as flexible electrode for both lithium and sodium storage. ACS Appl. Mater. Interfaces 2015, 7, 27804–27809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, C.M. Mesh-structured N-doped graphene@Sb2Se3 hybrids as an anode for large capacity sodium-ion batteries. J. Colloid Interface Sci. 2016, 488, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.S.; Tang, Y. Incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Lu, J.; Nan, C.; Li, L.; Peng, Q.; Li, Y. Flexible SnS nanobelts: Facile synthesis, formation mechanism and application in Li-ion batteries. Nano Res. 2013, 6, 55–64. [Google Scholar] [CrossRef]
- Huggins, R.A. Lithium alloy negative electrodes. J. Power Sources 1999, 81–82, 13–19. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Ma, L.; Guo, L.; Li, Q.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Three-dimensional network of N-doped carbon ultrathin nanosheets with closely packed mesopores: Controllable synthesis and application in electrochemical energy storage. ACS Appl. Mater. Interfaces 2016, 8, 11720–11728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.C.; Tao, H.C.; Yang, X.L.; Zhang, L.L.; Ni, S.B. Synthesis of N-doped graphene/SnS composite and its electrochemical properties for lithium ion batteries. Ionics 2015, 21, 2735–2742. [Google Scholar] [CrossRef]
- Gou, X.L.; Chen, J.; Shen, P.W. Synthesis, characterization and application of SnSx (x = 1, 2) nanoparticles. Mater. Chem. Phys. 2005, 93, 557–566. [Google Scholar] [CrossRef]
- Li, Y.; Tu, J.P.; Huang, X.H.; Wu, H.M.; Yuan, Y.F. Net-like SnS/carbon nanocomposite film anode material for lithium ion batteries. Electrochem. Commun. 2007, 9, 49–53. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–71129. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhang, F.; Liang, H.; Alshareef, H.N. Layered SnS sodium ion battery anodes synthesized near room temperature. Nano Res. 2017, 10, 4368–4377. [Google Scholar] [CrossRef]
- Dutta, P.K.; Sen, U.K.; Mitra, S. Excellent electrochemical performance of tin monosulphide (SnS) as a sodium-ion battery anode. RSC Adv. 2014, 4, 43155–43159. [Google Scholar] [CrossRef]
- Zhou, T.F.; Pang, W.K.; Zhang, C.F.; Yang, J.P.; Chen, Z.X.; Liu, H.K.; Guo, Z.P. Enhanced sodium-ion battery performance by structural phase transition from two-dimensional hexagonal-SnS2 to orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, Q.; Zhu, X.; Jiang, F. Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2018, 8, 135. https://doi.org/10.3390/nano8030135
Zhou Y, Wang Q, Zhu X, Jiang F. Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials. 2018; 8(3):135. https://doi.org/10.3390/nano8030135
Chicago/Turabian StyleZhou, Yanli, Qi Wang, Xiaotao Zhu, and Fuyi Jiang. 2018. "Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries" Nanomaterials 8, no. 3: 135. https://doi.org/10.3390/nano8030135
APA StyleZhou, Y., Wang, Q., Zhu, X., & Jiang, F. (2018). Three-Dimensional SnS Decorated Carbon Nano-Networks as Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials, 8(3), 135. https://doi.org/10.3390/nano8030135