Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Analytical Procedures
2.3. Synthesis of Diblock PMAgala-b-PMAChol Amphiphiles
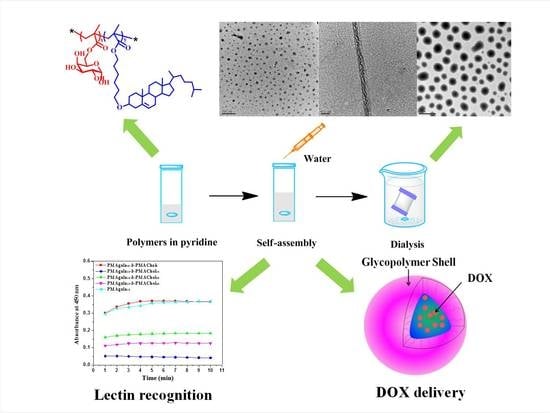
2.4. Self-Assembly of PMAgala-b-PMAChols in Solution
2.5. Lectin Recognition Assay
2.6. BSA Adsorption Assay
2.7. Preparation of the PMAgala-b-PMAChol/DOX Complex Aggregates
2.8. Cell Viability Assay
3. Results and Discussion
3.1. Synthesis and Characterization of the PMAgala-b-PMAChol Amphiphilic Copolymers
3.2. Self-Assembly of the PMAgala-b-PMAChol Amphiphiles in Pyridine/Water Mixed Solvent
3.3. Lectin Recognition of the PMAgala-b-PMAChol Aggregates
3.4. Serum Protein Adsorption of the PMAgala-b-PMAChol Aggregates
3.5. Intracellular Doxorubicin (DOX) Delivery by the PMAgala18-b-PMAChol/DOX Complex Aggregates
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sheng, R.; Luo, T.; Wang, Z.; Li, H.; Cao, A. Synthesis of diblock/statistical cationic glycopolymers with pendant galactose and lysine moieties: Gene delivery application and intracellular behaviors. J. Mater. Chem. B 2016, 4, 4696–4706. [Google Scholar] [CrossRef]
- Wang, Z.; Sheng, R.; Luo, T.; Sun, J.; Cao, A. Synthesis and self-assembly of diblock glycopolypeptide analogues PMAgala-b-PBLG as multifunctional biomaterials for protein recognition, drug delivery and hepatoma cell targeting. Polym. Chem. 2017, 8, 472–484. [Google Scholar] [CrossRef]
- Von der Ehe, C.; Weber, C.; Gottschaldt, M.; Schubert, U.S. Immobilized glycopolymers: Synthesis, methods and applications. Prog. Polym. Sci. 2016, 57, 64–102. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Li, F. Multiple morphologies of aggregates from block copolymers containing glycopolymer segments. Chem. Commun. 1999, 16, 1557–1558. [Google Scholar] [CrossRef]
- Pati, D.; Das, S.; Patil, N.G.; Parekh, N.; Anjum, D.H.; Dhaware, V.; Ambade, A.V.; Sen Gupta, S. Glycopolypeptide-based amphiphilic biocompatible star copolymers and their carbohydrate specific intracellular delivery. Biomacromolecules 2016, 17, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Ladmiral, V.; Semsarilar, M.; Canton, I.; Armes, S.P. Polymerization-induced self-assembly of galactose-functionalized biocompatible diblock copolymers for intracellular delivery. J. Am. Chem. Soc. 2013, 135, 13574–13581. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, C.; Polzer, F.; Lu, Y.; Chen, G.; Jiang, M. Glyco-inside micelles and vesicles directed by protection-deprotection chemistry. ACS Macro Lett. 2014, 3, 534–539. [Google Scholar] [CrossRef]
- Wu, X.; Su, L.; Chen, G.; Jiang, M. Deprotection-induced micellization of glycopolymers: Control of kinetics and morphologies. Macromolecules 2015, 48, 3705–3712. [Google Scholar] [CrossRef]
- Schlaad, H.; You, L.; Sigel, R.; Smarsly, B.; Heydenreich, M.; Mantion, A.; Masić, A. Glycopolymer vesicles with an asymmetric membrane. Chem. Commun. 2009, 1, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Bonduelle, C.; Thévenot, J.; Lecommandoux, S.; Heise, A. Biologically active polymersomes from amphiphilic glycopeptides. J. Am. Chem. Soc. 2012, 134, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Qu, K.; Rehman, A. Glycosylated conductive polymer: A multimodal biointerface for studying carbohydrate–protein interactions. Acc. Chem. Res. 2016, 49, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Yang, G.; Weiss, M.S.; Liu, Y.; Chen, G.; Jiang, M. Protein crystalline frameworks with controllable interpenetration directed by dual supramolecular interactions. Nat. Commun. 2014, 5, 4634. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Li, J.; Chen, G.; Jiang, M. Dual molecular recognition leading to a protein–polymer conjugate and further self-assembly. ACS Macro Lett. 2013, 2, 278–283. [Google Scholar] [CrossRef]
- Reynhout, I.C.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Synthesis of polymer-biohybrids: From small to giant surfactants. Acc. Chem. Res. 2009, 42, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Haraya, N.; Yamamichi, S. Chemical stimuli-responsive supramolecular hydrogel from amphiphilic tris-urea. Chem. Asian J. 2011, 6, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.R.S.; Chen, G.; Stenzel, M.H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polym. Chem. 2010, 1, 1392–1412. [Google Scholar] [CrossRef]
- Lu, J.; Fu, C.; Wang, S.; Tao, L.; Yan, L.; Haddleton, D.M.; Chen, G.; Wei, Y. From polymer sequence control to protein recognition: Synthesis, self-assembly and lectin binding. Macromolecules 2014, 47, 4676–4683. [Google Scholar] [CrossRef]
- Liau, W.T.; Bonduelle, C.; Brochet, M.; Lecommandoux, S.; Kasko, A.M. Synthesis, characterization, and biological interaction of glyconanoparticles with controlled branching. Biomacromolecules 2015, 16, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jia, Y.G.; Zhu, X. Glycopolymers bearing galactose and betulin: Synthesis, encapsulation, and lectin recognition. Biomacromolecules 2017, 18, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of nanomaterials in vivo: Blood circulation and organ clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, J.; Thomas, A.; OuYang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Williford, J.-M.; Santos, J.L.; Shyam, R.; Mao, H.Q. Shape control in engineering of polymeric nanoparticles for therapeutic delivery. Biomater. Sci. 2015, 3, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, J.; Tian, J.; Ge, Z.; Zhang, G.; Luo, K.; Liu, S. Polyprodrug amphiphiles: Hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 2013, 135, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Shrestha, R.; Clark, C.; Taylor, S.; Leonard, J.; Wooley, K.L. Multifunctional hierarchically assembled nanostructures as complex stage-wise dual-delivery systems for coincidental yet differential trafficking of siRNA and paclitaxel. Nano Lett. 2013, 13, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qu, W.; Pan, D.; Ren, Y.; Williford, J.-M.M.; Cui, H.; Luijten, E.; Mao, H. Plasmid-templated shape control of condensed DNA–block copolymer nanoparticles. Adv. Mater. 2013, 25, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Hartgerink, J.D.; Storrie, H.; Stendahl, J.C.; Stupp, S.I. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005, 1, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, B.; Esser, L.; Duong, H.T.; Basuki, J.S.; Boyer, C.; Davis, T.P. Polymerization-induced self-assembly (PISA)–control over the morphology of nanoparticles for drug delivery applications. Polym. Chem. 2014, 5, 350–355. [Google Scholar] [CrossRef]
- Cui, F.; Lin, J.; Li, Y.; Li, Y.; Wu, H.; Yu, F.; Jia, M.; Yang, X.; Wu, S.; Xie, L.; Ye, S.; Luo, F.; Hou, Z. Bacillus-shape design of polymer based drug delivery systems with janus-faced function for synergistic targeted drug delivery and more effective cancer. Mol. Pharm. 2015, 12, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Hinde, E.; Thammasiraphop, K.; Duong, H.T.T.; Yeow, J.; Karagoz, B.; Boyer, C.; Gooding, J.J.; Gaus, K. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat. Nanotechnol. 2016, 12, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Development of structural complexity by liquid-crystal self-assembly. Angew. Chem. Int. Ed. 2013, 52, 8828–8878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Z.; Li, M.; Sheng, R.; Luo, L.; Cao, A. Preparation and self-assembly of PHEMAChol-b-PBLG diblock copolymers bearing rigid liquid crystal cholesterol grafts. Acta Polym. Sin. 2016, 5, 667–678. [Google Scholar]
- Hosta-Rigau, L.; Zhang, Y.; Teo, B.M.; Postma, A.; Städler, B. Cholesterol-a biological compound as a building block in bionanotechnology. Nanoscale 2013, 5, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Ercole, F.; Whittaker, M.R.; Quinn, J.F.; Davis, T.P. Cholesterol modified self-assemblies and their application to nanomedicine. Biomacromolecules 2015, 16, 1886–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ahn, S.K.; Lakhman, R.K.; Gopinadhan, M.; Osuji, C.O.; Kasi, R.M. Tailoring crystallization behavior of PEO-based liquid crystalline block copolymers through variation in liquid crystalline content. Macromolecules 2011, 44, 3924–3934. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Venkataraman, S.; Sirat, S.B.M.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Lee, A.L.; Maune, H.T.; Hedrick, J.L.; Prabhu, V.M.; Yang, Y.Y. Formation of disk- and stacked-disk-like self-assembled morphologies from cholesterol-functionalized amphiphilic polycarbonate diblock copolymers. Macromolecules 2013, 46, 4839–4846. [Google Scholar] [CrossRef]
- Jia, L.; Albouy, P.A.; Di Cicco, A.; Cao, A.; Li, M.H. Self-assembly of amphiphilic liquid crystal block copolymers containing a cholesteryl mesogen: Effects of block ratio and solvent. Polymer. 2011, 52, 2565–2575. [Google Scholar] [CrossRef]
- Jia, L.; Liu, M.; Di Cicco, A.; Albouy, P.-A.; Brissault, B.; Penelle, J.; Boileau, S.; Barbier, V.; Li, M.H. Self-assembly of amphiphilic liquid crystal polymers obtained from a cyclopropane-1,1-dicarboxylate bearing a cholesteryl mesogen. Langmuir 2012, 28, 11215–11224. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Lévy, D.; Durand, D.; Impéror-Clerc, M.; Cao, A.; Li, M.H. Smectic polymer micellar aggregates with temperature-controlled morphologies. Soft Matter 2011, 7, 7395–7403. [Google Scholar] [CrossRef]
- Jia, L.; Cao, A.; Lévy, D.; Xu, B.; Albouy, P.A.; Xing, X.; Bowick, M.J.; Li, M.H. Smectic polymer vesicles. Soft Matter 2009, 5, 3446–3451. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Li, H.; Sun, J.; Sheng, R.; Luo, T.; Cao, A. Preparation of new amphiphilic liquid-crystal diblock copolymers bearing side-on cholesteryl mesogen and their self-aggregation. Acta Chim. Sin. 2013, 71, 351–359. [Google Scholar] [CrossRef]
- Sevimli, S.; Inci, F.; Zareie, H.M.; Bulmus, V. Well-defined cholesterol polymers with pH-controlled membrane switching activity. Biomacromolecules 2012, 13, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lei, Q.; Yang, B.; Jia, H.; Qiu, W.; Wang, X.; Zeng, X.; Zhuo, R.; Feng, J.; Zhang, X. Efficient nuclear drug translocation and improved drug efficacy mediated by acidity-responsive boronate-linked dextran/cholesterol nanoassembly. Biomaterials 2015, 52, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sevimli, S.; Sagnella, S.; Macmillan, A.; Whan, R.; Kavallaris, M.; Bulmus, V.; Davis, T.P. The endocytic pathway and therapeutic efficiency of doxorubicin conjugated cholesterol-derived polymers. Biomater. Sci. 2015, 3, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, T.; Sheng, R.; Li, H.; Sun, J.; Cao, A. Amphiphilic diblock terpolymer PMAgala-b-P(MAA-co-MAChol)s with attached galactose and cholesterol grafts and their intracellular pH-responsive doxorubicin delivery. Biomacromolecules 2016, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Luo, T.; Zhu, Y.; Li, H.; Sun, J.; Chen, S.; Sun, W.; Cao, A. The intracellular plasmid DNA localization of cationic reducible cholesterol-disulfide lipids. Biomaterials 2011, 32, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Luo, T.; Li, H.; Sun, J.; Wang, Z.; Cao, A. ‘Click’ synthesized sterol-based cationic lipids as gene carriers, and the effect of skeletons and headgroups on gene delivery. Bioorg. Med. Chem. 2013, 21, 6366–6377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, K.; Eisenberg, A. Ion-induced morphological changes in ‘crew-cut’ aggregates of amphiphilic dlock copolymers. Science 1996, 272, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, J.; Li, D.; Xiao, C.; Zhang, J.; He, C.; Zhuang, X.; Chen, X. Biocompatible reduction-responsive polypeptide micelles as nanocarriers for enhanced chemotherapy efficacy in vitro. J. Mater. Chem. B 2013, 1, 69–81. [Google Scholar] [CrossRef]
- Lv, S.; Li, M.; Tang, Z.; Song, W.; Sun, H.; Liu, H.; Chen, X. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomater. 2013, 9, 9330–9342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, C.; Pan, C. Galactose-based amphiphilic block copolymers: Synthesis, micellization, and bioapplication. Biomacromolecules 2013, 14, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Choucair, A.; Lavigueur, C.; Eisenberg, A. Polystyrene-b-poly(acrylic acid) vesicle size control using solution properties and hydrophilic block length. Langmuir 2004, 20, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, M.; Cameron, N.R.; Davis, B.G. Lectins: Tools for the molecular understanding of the glycocode. Org. Biomol. Chem. 2005, 3, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting—Strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Hong, D.J.; Bae, J.; Lee, M. Controlled self-assembly of carbohydrate conjugate rod–coil amphiphiles for supramolecular multivalent ligands. J. Am. Chem. Soc. 2005, 127, 16333–16337. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, C.; Li, F.; Ouyang, C.; Gao, Q.; Zheng, K. Nanoparticles vs. nanofibers: A comparison of two drug delivery systems on assessing drug release performance in vitro. Des. Monomers Polym. 2015, 18, 678–689. [Google Scholar] [CrossRef]









| Entry 1 | Sample | Monomer Conversion (%) 2 | Molecular Weight | PMAgala/PMAChol Ratio (wt %) | |||
|---|---|---|---|---|---|---|---|
| Mn, thero 3 (kg mol−1) | Mw, GPC 4 (kg mol−1) | Mn, GPC 4 (kg mol−1) | Mw/Mn 4 | ||||
| 1 | PMAIpGP18 | 91 | 6.37 | 5.56 | 4.95 | 1.10 | - |
| 2 | PMAIpGP18-b-PMAChol8 | 82 | 10.94 | 12.56 | 10.95 | 1.14 | 50/50 |
| 3 | PMAIpGP18-b-PMAChol24 | 96 | 19.74 | 20.84 | 16.23 | 1.28 | 25/75 |
| 4 | PMAIpGP18-b-PMAChol38 | 96 | 27.76 | 19.83 | 24.74 | 1.23 | 17/83 |
| 5 | PMAIpGP18-b-PMAChol48 | 95 | 32.83 | 36.19 | 31.23 | 1.15 | 14/86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Luo, T.; Cao, A.; Sun, J.; Jia, L.; Sheng, R. Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials 2018, 8, 136. https://doi.org/10.3390/nano8030136
Wang Z, Luo T, Cao A, Sun J, Jia L, Sheng R. Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials. 2018; 8(3):136. https://doi.org/10.3390/nano8030136
Chicago/Turabian StyleWang, Zhao, Ting Luo, Amin Cao, Jingjing Sun, Lin Jia, and Ruilong Sheng. 2018. "Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications" Nanomaterials 8, no. 3: 136. https://doi.org/10.3390/nano8030136
APA StyleWang, Z., Luo, T., Cao, A., Sun, J., Jia, L., & Sheng, R. (2018). Morphology-Variable Aggregates Prepared from Cholesterol-Containing Amphiphilic Glycopolymers: Their Protein Recognition/Adsorption and Drug Delivery Applications. Nanomaterials, 8(3), 136. https://doi.org/10.3390/nano8030136