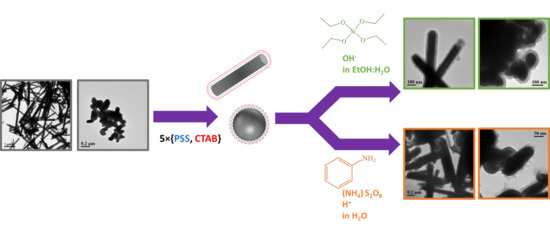
Preparation and Characterization of WS2@SiO2 and WS2@PANI Core-Shell Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Layer-by-Layer Surface Modification of WS2 Nanostructures
2.2. Preparation of WS2@SiO2 Nanocomposites
2.3. Preparation of WS2@PANI Nanocomposites
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Tenne, R. Inorganic nanotubes and fullerene-like nanoparticles. Nat. Nano 2006, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Jenei, I.Z.; Svahn, F.; Csillag, S. Correlation Studies of WS2 Fullerene-Like Nanoparticles Enhanced Tribofilms: A Scanning Electron Microscopy Analysis. Tribol. Lett. 2013, 51, 461–468. [Google Scholar] [CrossRef]
- Rapoport, L.; Lvovsky, M.; Lapsker, I.; Leshchinsky, W.; Volovik, Y.; Feldman, Y.; Tenne, R. Friction and wear of bronze powder composites including fullerene-like WS2 nanoparticles. Wear 2001, 249, 149–156. [Google Scholar] [CrossRef]
- Rapoport, L.; Lvovsky, M.; Lapsker, I.; Leshinsky, V.; Volovik, Y.; Feldman, Y.; Zak, A.; Tenne, R. Slow Release of Fullerene-Like WS2 Nanoparticles as a Superior Solid Lubrication Mechanism in Composite Matrices. Adv. Eng. Mater. 2001, 3, 71–75. [Google Scholar] [CrossRef]
- Kaplan-Ashiri, I.; Tenne, R. Mechanical Properties of WS2 Nanotubes. J. Clust. Sci. 2007, 18, 549–563. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Naffakh, M.; Díez-Pascual, A.M.; Ania, F.; Gómez-Fatou, M.A. Evaluating the Reinforcement of Inorganic Fullerene-like Nanoparticles in Thermoplastic Matrices by Depth-Sensing Indentation. J. Phys. Chem. C 2013, 117, 20936–20943. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.-X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low Cytotoxicity of Inorganic Nanotubes and Fullerene-Like Nanostructures in Human Bronchial Epithelial Cells: Relation to Inflammatory Gene Induction and Antioxidant Response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Asadi, V.; Jafari, S.H.; Khonakdar, H.A.; Häuβler, L.; Wagenknecht, U. Incorporation of inorganic fullerene-like WS2 into poly(ethylene succinate) to prepare novel biodegradable nanocomposites: A study on isothermal and dynamic crystallization. RSC Adv. 2016, 6, 4925–4935. [Google Scholar] [CrossRef]
- Silverman, T.; Naffakh, M.; Marco, C.; Ellis, G. Morphology and thermal properties of biodegradable poly(hydroxybutyrate-co-hydroxyvalerate)/tungsten disulphide inorganic nanotube nanocomposites. Mater. Chem. Phys. 2016, 170, 145–153. [Google Scholar] [CrossRef]
- Tahir, M.N.; Zink, N.; Eberhardt, M.; Therese, H.A.; Kolb, U.; Theato, P.; Tremel, W. Overcoming the Insolubility of Molybdenum Disulfide Nanoparticles through a High Degree of Sidewall Functionalization Using Polymeric Chelating Ligands. Angew. Chem. Int. Ed. 2006, 45, 4809–4815. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Zink, N.; Eberhardt, M.; Therese, H.A.; Faiss, S.; Janshoff, A.; Kolb, U.; Theato, P.; Tremel, W. Hierarchical Assembly of TiO2 Nanoparticles on WS2 Nanotubes Achieved Through Multifunctional Polymeric Ligands. Small 2007, 3, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Yella, A.; Therese, H.A.; Mugnaioli, E.; Panthöfer, M.; Khan, H.U.; Knoll, W.; Kolb, U.; Tremel, W. Synthesis of Hierarchically Grown ZnO@NT-WS2 Nanocomposites. Chem. Mater. 2009, 21, 5382–5387. [Google Scholar] [CrossRef]
- Tahir, M.N.; Yella, A.; Sahoo, J.K.; Natalio, F.; Kolb, U.; Jochum, F.; Theato, P.; Tremel, W. IF-ReS2 with Covalently Linked Porphyrin Antennae. Isr. J. Chem. 2010, 50, 500–505. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Tahir, M.N.; Yella, A.; Branscheid, R.; Kolb, U.; Tremel, W. Soluble IF-ReS2 Nanoparticles by Surface Functionalization with Terpyridine Ligands. Langmuir 2011, 27, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Tahir, M.N.; Yella, A.; Schladt, T.D.; Mugnaoli, E.; Kolb, U.; Tremel, W. Reversible Self-Assembly of Metal Chalcogenide/Metal Oxide Nanostructures Based on Pearson Hardness. Angew. Chem. Int. Ed. 2010, 49, 7578–7582. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Tahir, M.N.; Yella, A.; Schladt, T.D.; Pfeiffer, S.; Nakhjavan, B.; Mugnaioli, E.; Kolb, U.; Tremel, W. From Single Molecules to Nanoscopically Structured Materials: Self-Assembly of Metal Chalcogenide/Metal Oxide Nanostructures Based on the Degree of Pearson Hardness. Chem. Mater. 2011, 23, 3534–3539. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Tahir, M.N.; Hoshyargar, F.; Nakhjavan, B.; Branscheid, R.; Kolb, U.; Tremel, W. Molecular Camouflage: Making Use of Protecting Groups To Control the Self-Assembly of Inorganic Janus Particles onto Metal–Chalcogenide Nanotubes by Pearson Hardness. Angew. Chem. Int. Ed. 2011, 50, 12271–12275. [Google Scholar] [CrossRef] [PubMed]
- Shahar, C.; Zbaida, D.; Rapoport, L.; Cohen, H.; Bendikov, T.; Tannous, J.; Dassenoy, F.; Tenne, R. Surface Functionalization of WS2 Fullerene-like Nanoparticles. Langmuir 2009, 26, 4409–4414. [Google Scholar] [CrossRef] [PubMed]
- Shahar, C.; Levi, R.; Cohen, S.R.; Tenne, R. Gold Nanoparticles as Surface Defect Probes for WS2 Nanostructures. J. Phys. Chem. Lett. 2010, 1, 540–543. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Yadgarov, L.; Popovitz-Biro, R.; Lebedev, V.A.; Pinkas, I.; Rosentsveig, R.; Feldman, Y.; Goldt, A.E.; Goodilin, E.A.; Tenne, R. Decoration of WS2 Nanotubes and Fullerene-Like MoS2 with Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 2161–2169. [Google Scholar] [CrossRef]
- Ansari, A.A.; Singh, S.P.; Singh, N.; Malhotra, B.D. Synthesis of optically active silica-coated NdF3 core–shell nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhou, G.; Chen, S.; Wang, S. Synthesis and up-conversion photoluminescence properties of NaYF4:Yb3+, Er3+@sSiO2@mSiO2 nanoparticles. Opt. Mater. 2014, 36, 1443–1448. [Google Scholar] [CrossRef]
- Jin, C.; Kim, H.; Hong, C.; Kim, H.W.; Lee, C. Preparation and photoluminescence properties of silica-coated CuO nanowires. Appl. Phys. A 2010, 100, 151–157. [Google Scholar] [CrossRef]
- Ansari, A.A. Impact of surface coating on morphological, optical and photoluminescence properties of YF3:Tb3+ nanoparticles. Chin. Chem. Lett. 2017, 28, 651–657. [Google Scholar] [CrossRef]
- Ansari, A.A.; Alam, M.; Labis, J.P.; Alrokayan, S.A.; Shafi, G.; Hasan, T.N.; Syed, N.A.; Alshatwi, A.A. Luminescent mesoporous LaVO4:Eu3+ core-shell nanoparticles: Synthesis, characterization, biocompatibility and their cytotoxicity. J. Mater. Chem. 2011, 21, 19310–19316. [Google Scholar] [CrossRef]
- Ansari, A.A. Silica-modified luminescent LaPO4:Eu@LaPO4@SiO2 core/shell nanorods: Synthesis, structural and luminescent properties. Luminescence 2018, 33, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Das, H.; Arai, T.; Debnath, N.; Sakamoto, N.; Shinozaki, K.; Suzuki, H.; Wakiya, N. Impact of acidic catalyst to coat superparamagnetic magnesium ferrite nanoparticles with silica shell via sol–gel approach. Adv. Powder Technol. 2016, 27, 541–549. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Starchikov, S.S.; Gervits, N.E.; Korotkov, N.Y.; Dmitrieva, T.V.; Lin, C.-R.; Tseng, Y.-T.; Shih, K.-Y.; Lee, J.-S.; Wang, C.-C. Canted spin structure and the first order magnetic transition in CoFe2O4 nanoparticles coated by amorphous silica. J. Magn. Magn. Mater. 2016, 415, 13–19. [Google Scholar] [CrossRef]
- Tavares, D.S.; Daniel-da-Silva, A.L.; Lopes, C.B.; Silva, N.J.O.; Amaral, V.S.; Rocha, J.; Pereira, E.; Trindade, T. Efficient sorbents based on magnetite coated with siliceous hybrid shells for removal of mercury ions. J. Mater. Chem. A 2013, 1, 8134–8143. [Google Scholar] [CrossRef]
- Majeed, J.; Ramkumar, J.; Chandramouleeswaran, S.; Tyagi, A.K. Fe3O4@SiO2 core-shell nanoparticles: Synthesis, characterization and application in environmental remediation. AIP Conf. Proc. 2014, 1591, 605–607. [Google Scholar] [CrossRef]
- Gass, S.; Cohen, J.M.; Pyrgiotakis, G.; Sotiriou, G.A.; Pratsinis, S.E.; Demokritou, P. Safer Formulation Concept for Flame-Generated Engineered Nanomaterials. ACS Sustain. Chem. Eng. 2013, 1, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.-Y.; Franke, G. Effectiveness of amorphous silica encapsulation technology on welding fume particles and its impact on mechanical properties of welds. Mater. Des. 2014, 54, 79–86. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Z.; Xi, Y.; Zou, Q.; Li, X.; Wang, B.; Guo, X.; Liang, M.; Li, W.; Wang, M.; et al. Preparation of silica-coated ultrafine diamond and dispersion in ceramic matrix. Mater. Lett. 2013, 113, 134–137. [Google Scholar] [CrossRef]
- Konduru, N.V.; Jimenez, R.J.; Swami, A.; Friend, S.; Castranova, V.; Demokritou, P.; Brain, J.D.; Molina, R.M. Silica coating influences the corona and biokinetics of cerium oxide nanoparticles. Part. Fibre Toxicol. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.C.; Derk, R.; He, X.; Stueckle, T.A.; Cohen, J.; Pirela, S.V.; Demokritou, P.; Rojanasakul, Y.; Wang, L. Direct stimulation of human fibroblasts by nCeO2 in vitro is attenuated with an amorphous silica coating. Part. Fibre Toxicol. 2016, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Konduru, N.V.; Murdaugh, K.M.; Swami, A.; Jimenez, R.J.; Donaghey, T.C.; Demokritou, P.; Brain, J.D.; Molina, R.M. Surface modification of zinc oxide nanoparticles with amorphous silica alters their fate in the circulation. Nanotoxicology 2016, 10, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ostadhossein, A.; Kim, S.-Y.; Cubuk, E.D.; Qi, Y.; van Duin, A.C.T. Atomic Insight into the Lithium Storage and Diffusion Mechanism of SiO2/Al2O3 Electrodes of Lithium Ion Batteries: ReaxFF Reactive Force Field Modeling. J. Phys. Chem. A 2016, 120, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Synth. Met. 2001, 125, 23–42. [Google Scholar] [CrossRef]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Madheswari, D.; Mubarak Ali, M. Chemical formation, characterization and properties of polycarbazole. J. Appl. Polym. Sci. 2010, 116, 147–154. [Google Scholar] [CrossRef]
- Ates, M.; Uludag, N. Carbazole derivative synthesis and their electropolymerization. J. Solid State Electrochem. 2016, 20, 2599–2612. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Schopf, G.; Koßmehl, G. Properties of poly(thiophene)s. In Polythiophenes—Electrically Conductive Polymers; Springer: Berlin/Heidelberg, Germany, 1997; pp. 51–80. ISBN 978-3-540-68663-7. [Google Scholar]
- Lövenich, W. PEDOT-properties and applications. Polym. Sci. Ser. C 2014, 56, 135–143. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I. Polyaniline—A Conducting Polymer. In Materials Syntheses: A Practical Guide; Schubert, U., Hüsing, N., Laine, R.M., Eds.; Springer: Vienna, Austria, 2008; pp. 199–207. ISBN 978-3-211-75125-1. [Google Scholar]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Palaniappan, S.; Saravanan, C.; Amarnath, C.A.; Rao, V.J. Polyaniline Salts and Complexes as Catalyst in Bisindole Synthesis. Catal. Lett. 2004, 97, 77–81. [Google Scholar] [CrossRef]
- Chabukswar, V.V.; Handore, K.N.; Bhavsar, S.V.; Horne, A.S.; Dallavalle, S.; Gaikwad, V.; Mohite, K.C. Conducting Polyaniline is an Efficient Catalyst for Synthesis of 3,4-dihydropyrimidin-2-(1H)-one Derivative Under Solvent-Free Conditions. J. Macromol. Sci. Part A 2013, 50, 411–415. [Google Scholar] [CrossRef]
- Lai, B.; Tang, X.; Li, H.; Du, Z.; Liu, X.; Zhang, Q. Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biosens. Bioelectron. 2011, 28, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Dejaloud, M.; Jabbari, A. Nanostructured polyaniline-coated anode for improving microbial fuel cell power output. Chem. Pap. 2013, 67, 1096–1102. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Tang, Q.; Yue, G.; Lin, J.; Huang, M.; Meng, L. Bifacial dye-sensitized solar cells: A strategy to enhance overall efficiency based on transparent polyaniline electrode. Sci. Rep. 2014, 4, 4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Ahuja, T.; Kumar, D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens. Actuators B Chem. 2009, 136, 275–286. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Sanad, S.H.; Ismail, A.A. Coatings of Conducting Polymers for Corrosion Protection of Mild Steel. Silicon 2017, 9, 901–915. [Google Scholar] [CrossRef]
- De Barros, R.A.; Martins, C.R.; de Azevedo, W.M. Writing with conducting polymer. Synth. Met. 2005, 155, 35–38. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207–282. [Google Scholar] [CrossRef] [PubMed]
- Modak, P.; Kondawar, S.B.; Nandanwar, D.V. Synthesis and Characterization of Conducting Polyaniline/Graphene Nanocomposites for Electromagnetic Interference Shielding. Procedia Mater. Sci. 2015, 10, 588–594. [Google Scholar] [CrossRef]
- Sheng, X.; Cai, W.; Zhong, L.; Xie, D.; Zhang, X. Synthesis of Functionalized Graphene/Polyaniline Nanocomposites with Effective Synergistic Reinforcement on Anticorrosion. Ind. Eng. Chem. Res. 2016, 55, 8576–8585. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube–polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Awasthi, K. Polyaniline–Carbon Nanotube Composites: Preparation Methods, Properties, and Applications. Polym. Plast. Technol. Eng. 2017, 0, 1–28. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Sapurina, I.; Ćirić-Marjanović, G. The oxidation of aniline with silver nitrate to polyaniline–silver composites. Polymer 2009, 50, 50–56. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Rub, M.A.; Azum, N.; Khan, A.A.P.; Khan, S.B.; Rahman, M.M.; Khan, I. Synthesis, characterization of silver nanoparticle embedded polyaniline tungstophosphate-nanocomposite cation exchanger and its application for heavy metal selective membrane. Compos. Part B Eng. 2013, 45, 1486–1492. [Google Scholar] [CrossRef]
- Soni, A.; Pandey, C.M.; Solanki, S.; Sumana, G. One-pot synthesis of a polyaniline-gold nanocomposite and its enhanced electrochemical properties for biosensing applications. RSC Adv. 2015, 5, 45767–45774. [Google Scholar] [CrossRef]
- Wang, W.; Sun, S.; Gu, S.; Shen, H.; Zhang, Q.; Zhu, J.; Wang, L.; Jiang, W. One-pot fabrication and thermoelectric properties of Ag nanoparticles-polyaniline hybrid nanocomposites. RSC Adv. 2014, 4, 26810–26816. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline–TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth. Met. 2007, 157, 205–213. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Javadian, H.; Angaji, M.T.; Naushad, M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes. J. Ind. Eng. Chem. 2014, 20, 3890–3900. [Google Scholar] [CrossRef]
- Ramesan, M.T. Synthesis, characterization, and properties of new conducting polyaniline/copper sulfide nanocomposites. Polym. Eng. Sci. 2014, 54, 438–445. [Google Scholar] [CrossRef]
- Ghoswami, M.; Ghosh, R.; Chakraborty, G.; Gupta, K.; Meikap, A.K. Optical and electrical properties of polyaniline-cadmium sulfide nanocomposite. Polym. Compos. 2011, 32, 2017–2027. [Google Scholar] [CrossRef]
- Batool, A.; Kanwal, F.; Riaz, S.; Abbas, A.; Naseem, S. Novel Method to Synthesize Conducting Polyaniline/nickle Sulphide Nanocomposite Films and the Study of their Structural and Electrical Properties. Mater. Today Proc. 2015, 2, 5201–5204. [Google Scholar] [CrossRef]
- Hu, L.; Ren, Y.; Yang, H.; Xu, Q. Fabrication of 3D Hierarchical MoS2/Polyaniline and MoS2/C Architectures for Lithium-Ion Battery Applications. ACS Appl. Mater. Interfaces 2014, 6, 14644–14652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, P.; Wen, F.; Huang, C.; Wang, H. Construction of polyaniline/molybdenum sulfide nanocomposite: Characterization and its electrocatalytic performance on nitrite. Ionics 2016, 22, 1095–1102. [Google Scholar] [CrossRef]
- Lane, B.C.S.; Bissessur, R.; Abd-El-Aziz, A.S.; Alsaedi, W.H.; Dahn, D.C.; McDermott, E.; Martin, A. Exfoliated Nanocomposites Based on Polyaniline and Tungsten Disulfide. In Conducting Polymers; Yilmaz, F., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Voldman, A.; Zbaida, D.; Cohen, H.; Leitus, G.; Tenne, R. A Nanocomposite of Polyaniline/Inorganic Nanotubes. Macromol. Chem. Phys. 2013, 214, 2007–2015. [Google Scholar] [CrossRef]
- Chang, S.-H.; Tsai, Y.-T.; Li, G.-A.; Jheng, S.-L.; Kao, T.-L.; Tuan, H.-Y. Uniform silica coating of isoprene-passivated germanium nanowires via Stober method. RSC Adv. 2014, 4, 40146–40151. [Google Scholar] [CrossRef]
- Verraedt, E.; Pendela, M.; Adams, E.; Hoogmartens, J.; Martens, J.A. Controlled release of chlorhexidine from amorphous microporous silica. J. Control. Release 2010, 142, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Silica-Coating and Hydrophobation of CTAB-Stabilized Gold Nanorods. Chem. Mater. 2006, 18, 2465–2467. [Google Scholar] [CrossRef]
- Graf, C.; Vossen, D.L.J.; Imhof, A.; van Blaaderen, A. A General Method to Coat Colloidal Particles with Silica. Langmuir 2003, 19, 6693–6700. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Fibikar, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; De Cola, L. Microcontainers with Fluorescent Anisotropic Zeolite L Cores and Isotropic Silica Shells. Angew. Chem. Int. Ed. 2009, 48, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qin, P.; Luo, M.; Shao, E.; Zhao, H.; Yang, X.; Wang, Y.; Shen, H.; Jiao, Z.; Wu, M. Mesoporous silica coating on carbon nanotubes: Layer-by-layer method. Langmuir 2013, 29, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Stejskal, J.; Wang, J. Towards directional assembly of hierarchical structures: Aniline oligomers as the model precursors. Nanoscale 2013, 5, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tomšík, E.; Wang, J.; Morávková, Z.; Zhigunov, A.; Stejskal, J.; Trchová, M. Self-Assembly of Aniline Oligomers. Chem. Asian J. 2013, 8, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Simić, D.; Stojanović, D.B.; Kojović, A.; Dimić, M.; Totovski, L.; Uskoković, P.S.; Aleksić, R. Inorganic fullerene-like IF-WS2/PVB nanocomposites of improved thermo-mechanical and tribological properties. Mater. Chem. Phys. 2016, 184, 335–344. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Afzal Kamboh, M.; Rashidi Nodeh, H.; Mohamad, S. Synthesis of Polyaniline-Coated Graphene Oxide@SrTiO3 Nanocube Nanocomposites for Enhanced Removal of Carcinogenic Dyes from Aqueous Solution. Polymers 2016, 8, 305. [Google Scholar] [CrossRef]
- Choudhury, A. Polyaniline/silver nanocomposites: Dielectric properties and ethanol vapour sensitivity. Sens. Actuators B Chem. 2009, 138, 318–325. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Prijic, S.; Scancar, J.; Romih, R.; Cemazar, M.; Bregar, V.B.; Znidarsic, A.; Sersa, G. Increased Cellular Uptake of Biocompatible Superparamagnetic Iron Oxide Nanoparticles into Malignant Cells by an External Magnetic Field. J. Membr. Biol. 2010, 236, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Hessien, M.M.; Rashad, M.M.; Zaky, R.R.; Abdel-Aal, E.A.; El-Barawy, K.A. Controlling the synthesis conditions for silica nanosphere from semi-burned rice straw. Mater. Sci. Eng. B 2009, 162, 14–21. [Google Scholar] [CrossRef]
- Schmid, A.; Fujii, S.; Armes, S.P. Synthesis of Micrometer-Sized Silica-Stabilized Polystyrene Latex Particles. Langmuir 2005, 21, 8103–8105. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.J.; Amalvy, J.I.; Barthet, C.; Armes, S.P.; Greaves, S.J.; Watts, J.F.; Wiese, H. Surface characterization of vinyl polymer-silica colloidal nanocomposites using X-ray photoelectron spectroscopy. J. Mater. Chem. 2002, 12, 697–702. [Google Scholar] [CrossRef]
- Alila, S.; Boufi, S.; Belgacem, M.N.; Beneventi, D. Adsorption of a Cationic Surfactant onto Cellulosic Fibers I. Surface Charge Effects. Langmuir 2005, 21, 8106–8113. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and Comparison of Mesoporous Silica Particles for Optimized Drug Delivery. Nanomater. Nanotechnol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Yan, F.; Sun, L.; Li, F.; Zhuang, J.; Wang, H.; Yang, W. Mesoporous silica-coated superparamagnetic particles prepared by pseudomorphic transformation and their application in purification of plasmid DNA. J. Nanopart. Res. 2011, 13, 6613–6620. [Google Scholar] [CrossRef]
- De Araújo, A.C.V.; de Oliveira, R.J.; Júnior, S.A.; Rodrigues, A.R.; Machado, F.L.A.; Cabral, F.A.O.; de Azevedo, W.M. Synthesis, characterization and magnetic properties of polyaniline-magnetite nanocomposites. Synth. Met. 2010, 160, 685–690. [Google Scholar] [CrossRef]
- Lin, D.S.; Yang, S.M. EPR Studies of Poly(o-Phenetidine)-Poly(Styrenesulfonic Acid) and Poly(2-Ethylaniline)-Poly(Styrenesulfonic Acid) Complexes. J. Chin. Chem. Soc. 2004, 51, 1029–1035. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.-L.; Feng, J.; Jing, X.-L. Polyaniline micro-/nanostructures: Morphology control and formation mechanism exploration. Chem. Pap. 2013, 67, 876–890. [Google Scholar] [CrossRef]
- Kahol, P.K.; Pinto, N.J. An EPR investigation of electrospun polyaniline-polyethylene oxide blends. Synth. Met. 2004, 140, 269–272. [Google Scholar] [CrossRef]
- Krinichnyi, V.I. Multi Frequency EPR Spectroscopy of Conjugated Polymers and Their Nanocomposites. In Multi Frequency EPR Spectroscopy of Conjugated Polymers and Their Nanocomposites; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315349626. [Google Scholar]
- Green, U.; Aizenshtat, Z.; Ruthstein, S.; Cohen, H. Stable radicals formation in coals undergoing weathering: Effect of coal rank. Phys. Chem. Chem. Phys. 2012, 14, 13046–13052. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.; Palaniappan, S.; Sathyanarayana, D.N. Electron paramagnetic resonance and conductivity studies on poly(m-toluidine) salts and their bases. Synth. Met. 1994, 66, 129–134. [Google Scholar] [CrossRef]
- Rao, P.S.; Sathyanarayana, D.N. Electron spin resonance spectroscopy and electrical conductivity studies on some polyaniline salts and their bases. Indian J. Chem. 2004, 43A, 1377–1384. [Google Scholar]
- De Castro, E.G.; Zarbin, A.J.G.; de Oliveira, H.P.; Galembeck, A. Novel flexible, freestanding and transparent organic/inorganic hybrid materials formed between polyaniline and polyphosphate gel. Synth. Met. 2004, 146, 57–62. [Google Scholar] [CrossRef]
- Gupta, S.K.; Luthra, V.; Singh, R. Electrical transport and EPR investigations: A comparative study for dc conduction mechanism in monovalent and multivalent ions doped polyaniline. Bull. Mater. Sci. 2012, 35, 787–794. [Google Scholar] [CrossRef]
- Dyson, F.J. Electron Spin Resonance Absorption in Metals. II. Theory of Electron Diffusion and the Skin Effect. Phys. Rev. 1955, 98, 349–359. [Google Scholar] [CrossRef]
- Popovych, V.; Bester, M.; Stefaniuk, I.; Kuzma, M. Dyson line and modified Dyson line in the EPR measurements. Nukleonika 2015, 60, 385. [Google Scholar] [CrossRef]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sade, H.; Lellouche, J.-P. Preparation and Characterization of WS2@SiO2 and WS2@PANI Core-Shell Nanocomposites. Nanomaterials 2018, 8, 156. https://doi.org/10.3390/nano8030156
Sade H, Lellouche J-P. Preparation and Characterization of WS2@SiO2 and WS2@PANI Core-Shell Nanocomposites. Nanomaterials. 2018; 8(3):156. https://doi.org/10.3390/nano8030156
Chicago/Turabian StyleSade, Hagit, and Jean-Paul Lellouche. 2018. "Preparation and Characterization of WS2@SiO2 and WS2@PANI Core-Shell Nanocomposites" Nanomaterials 8, no. 3: 156. https://doi.org/10.3390/nano8030156
APA StyleSade, H., & Lellouche, J. -P. (2018). Preparation and Characterization of WS2@SiO2 and WS2@PANI Core-Shell Nanocomposites. Nanomaterials, 8(3), 156. https://doi.org/10.3390/nano8030156