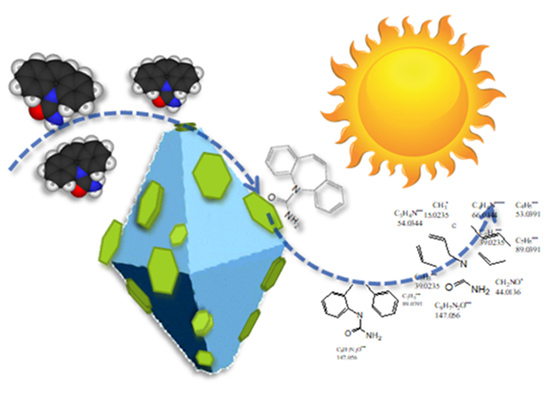
Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of MoOx/TiO2 Samples
2.1.2. Samples Activation and Sulfidation
2.2. Methods
2.2.1. Carbamazepine Photodegradation Tests
Irradiation Procedures
Analytical Procedures
3. Results and Discussion
3.1. Morphology and Structure of Samples
3.2. Optical and Surface Properties of Samples
3.3. Photocatalytic Activity
Carbamazepine Transformation Products’ Investigation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, J.; Yu, M. Hierarchical photocatalysis. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Sajan, C.P.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Cao, S. TiO2 nanosheets with exposed (001) facets for photocatalytic applications. Nano Res. 2016, 9, 3–27. [Google Scholar] [CrossRef]
- Puga, A. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Cravanzola, S.; Muscuso, L.; Cesano, F.; Agostini, G.; Damin, A.; Scarano, D.; Zecchina, A. MoS2 nanoparticles decorating titanate-nanotube surfaces: Combined microscopy, spectroscopy, and catalytic studies. Langmuir 2015, 31, 5469–5478. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; Pastor, E.; Kafizas, A.; Durrant, J.R. Evaluation of surface state mediated charge recombination in anatase and rutile tio2. J. Phys. Chem. Lett. 2016, 7, 3742–3746. [Google Scholar] [CrossRef] [PubMed]
- Patrocinio, A.O.T.; Schneider, J.; França, M.D.; Santos, L.M.; Caixeta, B.P.; Machado, A.E.H.; Bahnemann, D.W. Charge carrier dynamics and photocatalytic behavior of TiO2 nanopowders submitted to hydrothermal or conventional heat treatment. RSC Adv. 2015, 5, 70536–70545. [Google Scholar] [CrossRef] [Green Version]
- Cesano, F.; Agostini, G.; Scarano, D. Nanocrystalline TiO2 micropillar arrays grafted on conductive glass supports: Microscopic and spectroscopic studies. Thin Solid Film 2015, 590, 200–206. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Damin, A.; Agostini, G.; Usseglio, S.; Vitillo, J.G.; Lamberti, C.; Spoto, G.; Scarano, D.; Zecchina, A. Oriented TiO2 nanostructured pillar arrays: Synthesis and characterization. Adv. Mater. 2008, 20, 3342–3348. [Google Scholar] [CrossRef]
- Cesano, F.; Pellerej, D.; Scarano, D.; Ricchiardi, G.; Zecchina, A. Radially organized pillars of TiO2 nanoparticles: Synthesis, characterization and photocatalytic tests. J. Photochem. Photobiol. A 2012, 242, 51–58. [Google Scholar] [CrossRef]
- Wu, X.; Fang, S.; Zheng, Y.; Sun, J.; Lv, K. Thiourea-modified TiO2 nanorods with enhanced photocatalytic activity. Molecules 2016, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansaric, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (n-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Barkul, R.P.; Patil, M.K.; Patil, S.M.; Shevale, V.B.; Delekar, S.D. Sunlight-assisted photocatalytic degradation of textile effluent and rhodamine b by using iodine doped TiO2 nanoparticles. J. Photochem. Photobiol. A 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Hong, X.; Wang, Z.; Cai, W.; Lu, F.; Zhang, J.; Yang, Y.; Ma, N.; Liu, Y. Visible-light-activated nanoparticle photocatalyst of iodine-doped titanium dioxide. Chem. Mater. 2005, 17, 1548–1552. [Google Scholar] [CrossRef]
- Dozzi, M.V.; D’Andrea, C.; Ohtani, B.; Valentini, G.; Selli, E. Fluorine-doped TiO2 materials: Photocatalytic activity vs time-resolved photoluminescence. J. Phys. Chem. C 2013, 117, 25586–25595. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Ma, R.; Xue, Y.; Zheng, S. Fluorine doped anatase tio2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater. 2017, 243, 281–290. [Google Scholar] [CrossRef]
- Cravanzola, S.; Jain, S.M.; Cesano, F.; Damin, A.; Scarano, D. Development of a multifunctional TiO2/MWCNT hybrid composite grafted on a stainless steel grating. RSC Adv. 2015, 5, 103255–103264. [Google Scholar]
- Rasoulnezhad, H.; Kavei, G.; Ahmadi, K.; Rahimipour, M.R. Combined sonochemical/CVD method for preparation of nanostructured carbon-doped TiO2 thin film. Appl. Surf. Sci. 2017, 408, 1–10. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef] [PubMed]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Uddin, M.J.; Daramola, D.E.; Velasquez, E.; Dickens, T.J.; Yan, J.; Hammel, E.; Okoli, O.I. A high efficiency 3D photovoltaic microwire with carbon nanotubes (CNT)-quantum dot (QD) hybrid interface. PSS-RRL 2014, 8, 898–903. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Sulfur doped anatase TiO2 single crystals with a high percentage of {0 0 1} facets. J. Colloid Interface Sci. 2010, 349, 477–483. [Google Scholar] [CrossRef] [PubMed]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of s-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-doped TiO2: Structure and surface properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Piovano, A.; Agostini, G.; Rahman, M.M.; Groppo, E.; Bonino, F.; Scarano, D.; Lamberti, C.; Bordiga, S.; et al. Model oxide supported MoS2 HDS catalysts: Structure and surface properties. Catal. Sci. Technol. 2011, 1, 123–126. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Carbon domains on MoS2/TiO2 system via catalytic acetylene oligomerization: Synthesis, structure, and surface properties. Front. Chem. 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Tapoda, H.; Thampi, R.; Gratzel, M. Reduction of carbon oxides using supported molibdenum sulphide catalysts. In Recent Developments in Catalysis: Theory and Practice; Viswanathan, B., Pillai, C.N., Eds.; Narosa Publishing House: New Delhi, India, 1991; Volume 22, pp. 244–251. [Google Scholar]
- Sabarinathan, M.; Harish, S.; Archana, J.; Navaneethan, M.; Ikeda, H.; Hayakawa, Y. Highly efficient visible-light photocatalytic activity of MoS2–TiO2 mixtures hybrid photocatalyst and functional properties. RSC Adv. 2017, 7, 24754–24763. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, vibrational, and structural properties of MoS2 nanoparticles obtained by exfoliation and fragmentation via ultrasound cavitation in isopropyl alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, N.; Wei, C.; Zhou, J.; He, F.; Shi, C.; He, C.; Liu, E. Multi-functional integration of pore P25@C@MoS2 core-double shell nanostructures as robust ternary anodes with enhanced lithium storage properties. Appl. Surf. Sci. 2017, 401, 232–240. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Yu, Y.; Wang, S. Synthesis of core-shell TiO2@MoS2 composites for lithium-ion battery anodes. J. Alloys Compd. 2016, 689, 460–467. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium ion batteries. New J. Chem. 2015, 39, 683–688. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic h2 evolution on MoS2–TiO2 catalysts synthesized via mechanochemistry. Phys. Chem. Chem. Phys. 2015, 17, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Sun, J.D. Synthesis of nano-MoS2/TiO2 composite and its catalytic degradation effect on methyl orange. J. Mater. Sci. 2010, 45, 2640–2648. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Lu, Z.; Jin, Z.; Wang, X.; Xu, G.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; et al. Crystal face regulating MoS2/TiO2 (001) heterostructure for high photocatalytic activity. J. Alloys Compd. 2016, 688, 840–848. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. Uv-a and solar photodegradation of ibuprofen and carbamazepine catalyzed by Tio2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Minero, C. Fate of selected pharmaceuticals in river waters. Environ. Sci. Pollut. Res. 2013, 20, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Brar, S.K.; Daghrir, R.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y.; Drogui, P. Photocatalytic degradation of carbamazepine in wastewater by using a new class of whey-stabilized nanocrystalline TiO2 and ZnO. Sci. Total Environ. 2014, 485–486, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.P.T.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Jaume Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from cuernavaca, morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Deiana, C.; Minella, M.; Tabacchi, G.; Maurino, V.; Fois, E.; Martra, G. Shape-controlled tio2 nanoparticles and TiO2 P25 interacting with co and H2O2 molecular probes: A synergic approach for surface structure recognition and physico-chemical understanding. Phys. Chem. Chem. Phys. 2013, 15, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, X.; Zhou, W.; Liu, Z.; Xie, G.; Wang, Y.; Du, Y. High quality sulfur-doped titanium dioxide nanocatalysts with visible light photocatalytic activity from non-hydrolytic thermolysis synthesis. Inorg. Chem. Front. 2014, 1, 521–525. [Google Scholar] [CrossRef]
- Deiana, C.; Fois, E.; Martra, G.; Narbey, S.; Pellegrino, F.; Tabacchi, G. On the simple complexity of carbon monoxide on oxide surfaces: Facet-specific donation and backdonation effects revealed on TiO2 anatase nanoparticles. ChemPhysChem 2016, 17, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.F.; Lien, C.F.; Shieh, D.L.; Chen, M.T.; Lin, J.L. Ftir study of adsorption and photoassisted oxygen isotopic exchange of carbon monoxide, carbon dioxide, carbonate, and formate on TiO2. J. Phys. Chem. B 2002, 106, 11240–11245. [Google Scholar] [CrossRef]
- Huang, W.-F.; Chen, H.-T.; Lin, M.C. Density functional theory study of the adsorption and reaction of H2S on TiO2 rutile (110) and anatase (101) surface. J. Phys. Chem. C 2009, 113, 20411–20420. [Google Scholar] [CrossRef]
- Junkaew, A.; Maitarad, P.; Arróyave, R.; Kungwan, N.; Zhang, D.; Shi, L.; Namuangruk, S. The complete reaction mechanism of H2S desulfurization on an anatase TiO2 (001) surface: A density functional theory investigation. Catal. Sci. Technol. 2017, 7, 356–365. [Google Scholar] [CrossRef]
- Yanxin, C.; Yi, J.; Wenzhao, L.; Rongchao, J.; Shaozhen, T.; Wenbin, H. Adsorption and interaction of H2S/SO2 on TiO2. Catal. Today 1999, 50, 39–47. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Baiocchi, C. Identification of the unknown transformation products derivedfrom Clarithromycin and carbamazepine using liquidchromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Leung, D.Y.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Yu, J.C.; Lin, J.; Yu, J.; Li, P. Preparation and photocatalytic behavior of MoS2 and WS2 nanocluster sensitized TiO2. Langmuir 2004, 20, 5865–5869. [Google Scholar] [CrossRef] [PubMed]






| [M + H]+ | Name | tR (min) | TiO2 P25 (Area) | Sulfided-TiO2 (Area) | MoS2/TiO2 (Area) |
|---|---|---|---|---|---|
| 237.1025 | CBZ | 21.33 | 1.07 × 109 | 1.17 × 109 | 8.71 × 108 |
| 253.0977 | 253-A | 14.77 | 8.39 × 106 | n.d. | n.d. |
| 253.0977 | 253-B | 17.30 | 5.37 × 107 | 2.89 × 107 | 5.51 × 107 |
| 253.0977 | 253-C | 18.34 | 5.41 × 107 | 3.01 × 107 | 5.22 × 107 |
| 253.0977 | 253-D | 19.08 | 6.98 × 106 | 2.19 × 106 | 3.60 × 106 |
| 251.0891 | 251 | 16.95 | 6.26 × 107 | 3.16 × 107 | 8.90 × 107 |
| 269.0935 | 269-A | 15.57 | n.d. | n.d. | 3.51 × 106 |
| 269.0935 | 269-B | 16.79 | 5.15 × 106 | n.d. | 5.02 × 106 |
| 267.0786 | 267-A | 15.25 | 1.28 × 106 | 6.26 × 105 | 2.29 × 106 |
| 267.0786 | 267-B | 16.49 | 1.36 × 106 | 1.98 × 106 | 3.37 × 106 |
| 267.0786 | 267-C | 18.51 | 6.24 × 105 | n.d. | n.d. |
| 271.1081 | 271 | 14.77 | 6.62 × 106 | n.d. | n.d. |
| 224.0710 | 224 | 22.40 | 8.19 × 106 | n.d. | n.d. |
| Sample | SBET (m2/g) |
|---|---|
| TiO2 | 55 |
| sulfided TiO2 | 38 |
| MoS2/TiO2 | 37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravanzola, S.; Sarro, M.; Cesano, F.; Calza, P.; Scarano, D. Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials 2018, 8, 207. https://doi.org/10.3390/nano8040207
Cravanzola S, Sarro M, Cesano F, Calza P, Scarano D. Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials. 2018; 8(4):207. https://doi.org/10.3390/nano8040207
Chicago/Turabian StyleCravanzola, Sara, Marco Sarro, Federico Cesano, Paola Calza, and Domenica Scarano. 2018. "Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine" Nanomaterials 8, no. 4: 207. https://doi.org/10.3390/nano8040207
APA StyleCravanzola, S., Sarro, M., Cesano, F., Calza, P., & Scarano, D. (2018). Few-Layer MoS2 Nanodomains Decorating TiO2 Nanoparticles: A Case Study for the Photodegradation of Carbamazepine. Nanomaterials, 8(4), 207. https://doi.org/10.3390/nano8040207