Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Nanoparticles
2.2. Growth Kinetics
2.3. Acute Toxicity 4-h Test
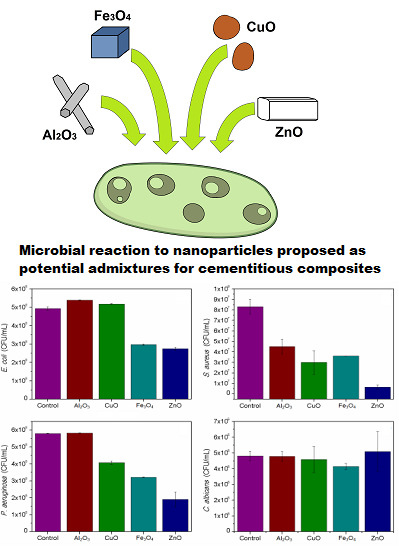
2.4. Toxicity in 24-h Test
2.5. Biofilm Formation Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Physiochemical Evaluation of Nanomaterials
4.3. Preparation of Nanomaterials for Microbiological Studies
4.3.1. Growth Kinetics
4.3.2. Toxicity Studies
4.3.3. Influence on Biofilm Formation
4.3.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.-S. Nano-TiO2-based architectural mortar for NO removal and bacteria inactivation: Influence of coating and weathering conditions. Cem. Concr. Compos. 2013, 36, 101–108. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Ou, J. Smart and Multifunctional Concrete toward Sustainable Infrastructures; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-981-10-4348-2. [Google Scholar]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.-S. TiO2-based self-compacting glass mortar: Comparison of photocatalytic nitrogen oxide removal and bacteria inactivation. Build. Environ. 2012, 53, 1–6. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B Environ. 2018, 222, 200–208. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Photocatalytic roads: from lab tests to real scale applications. Eur. Transp. Res. Rev. 2013, 5, 79–89. [Google Scholar] [CrossRef]
- Amrhein, K.; Stephan, D. Principles and test methods for the determination of the activity of photocatalytic materials and their application to modified building materials. Photochem. Photobiol. Sci. 2011, 10, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.W.; An, Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Combarros, R.G.; Collado, S.; Díaz, M. Toxicity of titanium dioxide nanoparticles on Pseudomonas putida. Water Res. 2016, 90, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Ge, Y.; Chang, V.; Stoimenov, P.K.; Schimel, J.P.; Stucky, G.D.; Holden, P.A. Assessing interactions of hydrophilic nanoscale TiO2 with soil water. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Rauscher, H.; Rasmussen, K.; Sokull-Kluttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chemie-Ingenieur-Technik 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Horszczaruk, E.; Rucinska, T.; Nawrotek, P.; Mijowska, E. Characterization of mechanical and bactericidal properties of cement mortars containing waste glass aggregate and nanomaterials. Materials 2016. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Song, H.; So, H.; Soh, Y. Antifungal effects of cement mortars with two types of organic antifungal agents. Cem. Concr. Res. 2005, 35, 371–376. [Google Scholar] [CrossRef]
- So, H.; Jang, H.; Lee, B.; So, S. Antifungal performance of BFS mortar with various natural antifungal substances and their physical properties. Constr. Build. Mater. 2016, 108, 154–162. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.-H.J.; Nam, J.-W.; Phan, H.D.; Kim, J.-K. Development of anti-fungal mortar and concrete using Zeolite and Zeocarbon microcapsules. Cem. Concr. Compos. 2009, 31, 447–453. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Antimicrobial mortar surfaces for the improvement of hygienic conditions. J. Appl. Microbiol. 2010, 108, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.A.; Schimel, J.P.; Godwin, H.A. Five reasons to use bacteria when assessing manufactured nanomaterial environmental hazards and fates. Curr. Opin. Biotechnol. 2014, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Antibacterial SnO2 nanorods as efficient fillers of poly(propylene fumarate-co-ethylene glycol) biomaterials. Mater. Sci. Eng. C 2017, 78, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Barylak, M.; Mijowska, E. Investigating the interaction between Streptomyces sp. and titania/silica nanospheres. Water Air Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Xu, W.; Xie, W.; Huang, X.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.M.C.; Chan, C.M.N.; Guo, M.Y.; Leung, Y.H.; Djurišić, A.B.; Hu, X.; Chan, W.K.; Leung, F.C.C.; Tong, S.Y. Antibacterial and photocatalytic activity of TiO2 and ZnO nanomaterials in phosphate buffer and saline solution. Appl. Microbiol. Biotechnol. 2013, 97, 5565–5573. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Seo, J.-W.T.; Corr, D.; Hersam, M.C.; Shah, S.P. Dispersion of CaCO3 nanoparticles by sonication and surfactant treatment for application in fly ash–cement systems. Mater. Struct. 2014, 47, 1011–1023. [Google Scholar] [CrossRef]
- Korayem, A.H.; Tourani, N.; Zakertabrizi, M.; Sabziparvar, A.M.; Duan, W.H. A review of dispersion of nanoparticles in cementitious matrices: Nanoparticle geometry perspective. Constr. Build. Mater. 2017, 153, 346–357. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R. A review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Alrekabi, S.; Cundy, A.; Whitby, R.L.D.; Lampropoulos, A.; Savina, I. Effect of undensified silica fume on the dispersion of carbon nanotubes within a cementitious composite. J. Phys. Conf. Ser. 2017, 829. [Google Scholar] [CrossRef]
- Mendoza, O.; Sierra, G.; Tobón, J.I. Effect of the reagglomeration process of multi-walled carbon nanotubes dispersions on the early activity of nanosilica in cement composites. Constr. Build. Mater. 2014, 54, 550–557. [Google Scholar] [CrossRef]
- Mateos, R.; Vera, S.; Valiente, M.; Díez-Pascual, A.; San Andrés, M. Comparison of anionic, cationic and nonionic surfactants as dispersing agents for graphene based on the fluorescence of riboflavin. Nanomaterials 2017, 7, 403. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Brown, L.; Sanchez, F. Quantification of the re-agglomeration of carbon nanofiber aqueous dispersion in cement pastes and effect on the early age flexural response. Carbon 2016, 107, 482–500. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Bairoliya, S.; Parashar, A.; Chandrasekaran, N.; Mukherjee, A. Differential toxicity of Al2O3 particles on Gram-positive and Gram-negative sediment bacterial isolates from freshwater. Environ. Sci. Pollut. Res. 2016, 23, 12095–12106. [Google Scholar] [CrossRef] [PubMed]
- Käkinen, A.; Kahru, A.; Nurmsoo, H.; Kubo, A.L.; Bondarenko, O.M. Solubility-driven toxicity of CuO nanoparticles to Caco2 cells and Escherichia coli: Effect of sonication energy and test environment. Toxicol. In Vitro 2016, 36, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. JoVE 2011, 47. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.; Forbes, S.; McBain, A.J. Attenuated virulence and biofilm formation in Staphylococcus aureus following sublethal exposure to triclosan. Antimicrob. Agents Chemother. 2012, 56, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Alnajar, S.; Naushad, S.G.R. Genome based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 5575–5599. [Google Scholar] [CrossRef]
- Nawrotek, P.; Grygorcewicz, B.; Augustyniak, A. Changes in the taxonomy of γ-Proteobacteria, modification of the order Enterobacteriales and novel families within Enterobacterales ord. nov. Postep. Mikrobiol. 2017, 56, 465–469. [Google Scholar]
- Struk, M.; Grygorcewicz, B.; Nawrotek, P.; Augustyniak, A.; Konopacki, M.; Kordas, M.; Rakoczy, R. Enhancing effect of 50 Hz rotating magnetic field on induction of Shiga toxin-converting lambdoid prophages. Microb. Pathog. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Chrobak, D.; Błaszczak, B.; Binek, M. Multidrug resistance in Escherichia coli strains isolated from infections in dogs and cats in poland (2007–2013). Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2010, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.J. Derivations and Genotypes of Some Mutant Derivatives of Escherichia coli K-12, 2nd ed.; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, USA, 1996; ISBN 1555810845. [Google Scholar]
- NCBI Taxonomy Browser, Search Item “Escherichia coli”. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Undef&id=562&lvl=3&lin=f&keep=1&srchmode=1&unlock (accessed on 17 January 2018).
- Karcagi, I.; Draskovits, G.; Umenhoffer, K.; Fekete, G.; Kovács, K.; Méhi, O.; Balikó, G.; Szappanos, B.; Györfy, Z.; Fehér, T.; et al. Indispensability of Horizontally transferred genes and its impact on bacterial genome streamlining. Mol. Biol. Evol. 2016, 33, 1257–1269. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials 2018, 8, 212. https://doi.org/10.3390/nano8040212
Sikora P, Augustyniak A, Cendrowski K, Nawrotek P, Mijowska E. Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials. 2018; 8(4):212. https://doi.org/10.3390/nano8040212
Chicago/Turabian StyleSikora, Pawel, Adrian Augustyniak, Krzysztof Cendrowski, Paweł Nawrotek, and Ewa Mijowska. 2018. "Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials" Nanomaterials 8, no. 4: 212. https://doi.org/10.3390/nano8040212
APA StyleSikora, P., Augustyniak, A., Cendrowski, K., Nawrotek, P., & Mijowska, E. (2018). Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials, 8(4), 212. https://doi.org/10.3390/nano8040212