Self-Catalyzed CdTe Wires
Abstract
:1. Introduction
2. Materials and Methods
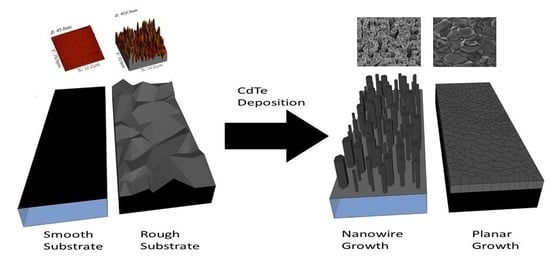
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Data Availability
References
- Wang, X.; Xu, Y.; Zhu, H.; Liu, R.; Wang, H.; Li, Q. Crystalline Te nanotube and Te nanorods-on-CdTe nanotube arrays on ITO via a ZnO nanorod templating-reaction. CrystEngComm 2011, 13, 2955–2959. [Google Scholar] [CrossRef]
- Ma, Y.; Jian, J.; Wu, R.; Sun, Y.; Li, J. Growth of single-crystalline ultra-long cadmium telluride micron-size wires via thermal evaporation. Micro Nano Lett. 2011, 6, 596–598. [Google Scholar] [CrossRef]
- Di Carlo, V.; Prete, P.; Dubrovskii, V.G.; Berdnikov, Y.; Lovergine, N. CdTe Nanowires by Au-Catalyzed Metalorganic Vapor Phase Epitaxy. Nano Lett. 2017, 17, 4075–4082. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.L.; Paulson, P.D.; Dutta, V. Thin-film solar cells: An overview. Prog. Photovolt. Res. Appl. 2004, 12, 69–92. [Google Scholar] [CrossRef]
- Consonni, V.; Rey, G.; Bonaiḿ, J.; Karst, N.; Doisneau, B.; Roussel, H.; Renet, S.; Bellet, D. Synthesis and physical properties of ZnO/CdTe core shell nanowires grown by low-cost deposition methods. Appl. Phys. Lett. 2011, 98, 96–99. [Google Scholar] [CrossRef]
- Lee, S.K.C.; Yu, Y.; Perez, O.; Puscas, S.; Kosel, T.H.; Kuno, M. Bismuth-assisted CdSe and CdTe nanowire growth on plastics. Chem. Mater. 2010, 22, 77–84. [Google Scholar] [CrossRef]
- Kret, S.; Szuszkiewicz, W.; Dynowska, E.; Domagala, J.; Aleszkiewicz, M.; Baczewski, L.T.; Petroutchik, A. MBE Growth and Properties of ZnTe- and CdTe-Based Nanowires. J. Korean Phys. Soc. 2008, 53, 3055–3063. [Google Scholar]
- Khalavka, Y.; Sönnichsen, C. Growth of gold tips onto hyperbranched CdTe nanostructures. Adv. Mater. 2008, 20, 588–591. [Google Scholar] [CrossRef]
- Davami, K.; Ghassemi, H.M.; Sun, X.; Yassar, R.S.; Lee, J.S.; Meyyappan, M. In Situ observation of morphological change in CdTe nano-and submicron wires. Nanotechnology 2011, 22, 435204. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, R.; Li, J.; Sun, Y.F.; Jian, J.K. CdTe nanosheets and pine-like hyperbranched nanostructures prepared by a modified film technique: Catalyst-assisted vacuum thermal evaporation. Mater. Lett. 2011, 65, 17–20. [Google Scholar] [CrossRef]
- Ruiz, C.M.; Saucedo, E.; Martínez, O.; Bermúdez, V. Hexagonal CdTe-like rods prompted from Bi2Te3 droplets. J. Phys. Chem. C 2007, 111, 5588–5591. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, S.B. Electronic Structures and defect physics of Cd-Based Semiconductors. In Proceedings of the Twenty-Eighth IEEE Photovoltaic Specialists Conference, Anchorage, AK, USA, 15–22 September 2000; IEEE: Piscataway, NJ, USA, 2000; pp. 483–486. [Google Scholar]
- Jin, X.; Kruszynska, M.; Parisi, J.; Kolny-Olesiak, J. Catalyst-free synthesis and shape control of CdTe nanowires. Nano Res. 2011, 4, 824–835. [Google Scholar] [CrossRef]
- Zhao, A.W.; Meng, G.W.; Zhang, L.D.; Gao, T.; Sun, S.H.; Pang, Y.T. Electrochemical synthesis of ordered CdTe nanowire arrays. Appl. Phys. A Mater. Sci. Process. 2003, 76, 537–539. [Google Scholar] [CrossRef]
- Neretina, S.; Hughes, R.A.; Britten, J.F.; Sochinskii, N.V.; Preston, J.S.; Mascher, P. Vertically aligned wurtzite CdTe nanowires derived from a catalytically driven growth mode. Nanotechnology 2007, 18, 275301. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, J.; Zhou, M.J.; Wang, H.; Xiao, X.D.; Li, Q. CdTe nanorods formation via nanoparticle self-assembly by thermal chemistry method. J. Cryst. Growth 2010, 312, 2310–2314. [Google Scholar] [CrossRef]
- Williams, B.L.; Major, J.D.; Bowen, L.; Phillips, L.; Zoppi, G.; Forbes, I.; Durose, K. Challenges and prospects for developing CdS/CdTe substrate solar cells on Mo foils. Sol. Energy Mater. Sol. Cells 2014, 124, 31–38. [Google Scholar] [CrossRef]
- Kranz, L.; Gretener, C.; Perrenoud, J.; Schmitt, R.; Pianezzi, F.; La Mattina, F.; Blösch, P.; Cheah, E.; Chirilă, A.; Fella, C.M.; Hagendorfer, H.; Jäger, T.; Nishiwaki, S.; et al. Doping of polycrystalline CdTe for high-efficiency solar cells on flexible metal foil. Nat. Commun. 2013, 4, 2306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Hsieh, Y.T.; Lai, C.M.; Hsu, H.R. Impact of Mo barrier layer on the formation of MoSe2 in Cu(In,Ga)Se2 solar cells. J. Alloys Compd. 2016, 661, 168–175. [Google Scholar] [CrossRef]
- Major, J.D.; Treharne, R.E.; Phillips, L.J.; Durose, K. A low-cost non-toxic post-growth activation step for CdTe solar cells. Nature 2014, 511, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Major, J.D.; Proskuryakov, Y.Y.; Durose, K.; Zoppi, G.; Forbes, I. Control of grain size in sublimation-grown CdTe, and the improvement in performance of devices with systematically increased grain size. Sol. Energy Mater. Sol. Cells 2010, 94, 1107–1112. [Google Scholar] [CrossRef]
- Major, J.D.; Proskuryakov, Y.Y.; Durose, K.; Green, S. Nucleation of CdTe thin films deposited by close-space sublimation under a nitrogen ambient. Thin Solid Films 2007, 515, 5828–5832. [Google Scholar] [CrossRef]
- Ambrosini, S.; Fanetti, M.; Grillo, V.; Franciosi, A.; Rubini, S. Vapor-liquid-solid and vapor-solid growth of self-catalyzed GaAs nanowires. AIP Adv. 2011, 1, 042142. [Google Scholar] [CrossRef]
- Venables, J.A. Introduction to Surface and Thin Film Processes; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780511755651. [Google Scholar]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Chen, S.W.; Wu, J.M. Nucleation mechanisms and their influences on characteristics of ZnO nanorod arrays prepared by a hydrothermal method. Acta Mater. 2011, 59, 841–847. [Google Scholar] [CrossRef]
- Ho, S.T.; Chen, K.C.; Chen, H.A.; Lin, H.Y.; Cheng, C.Y.; Lin, H.N. Catalyst-free surface-roughness-assisted growth of large-scale vertically aligned zinc oxide nanowires by thermal evaporation. Chem. Mater. 2007, 19, 4083–4086. [Google Scholar] [CrossRef]
- Mandl, B.; Stangl, J.; Hilner, E.; Zakharov, A.A.; Hillerich, K.; Dey, A.W.; Samuelson, L.; Bauer, G.; Deppert, K.; Mikkelsen, A. Growth mechanism of self-catalyzed group III-V nanowires. Nano Lett. 2010, 10, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.H.; Baptista, D.L.; Teo, K.B.K.; Lacerda, R.G.; Jefferson, D.A.; Milne, W.I. Controllable growth of vertically aligned zinc oxide nanowires using vapour deposition. Nanotechnology 2006, 17, 4811–4818. [Google Scholar] [CrossRef]
- Williams, B.L.; Major, J.D.; Bowen, L.; Keuning, W.; Creatore, M.; Durose, K. A Comparative Study of the Effects of Nontoxic Chloride Treatments on CdTe Solar Cell Microstructure and Stoichiometry. Adv. Energy Mater. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Mendis, B.G.; Bowen, L. Cathodoluminescence measurement of grain boundary recombination velocity in vapour grown p-CdTe. J. Phys. Conf. Ser. 2011, 326, 4–8. [Google Scholar] [CrossRef]





| Under Vacuum SLG/Mo | 30 Torr SLG/Mo | 30 Torr SLG/FTO/Mo | |
|---|---|---|---|
| C111 | 6.47 | 5.511 | 4.90 |
| C220 | 0.11 | 0.407 | 0.43 |
| C311 | 0.10 | 0.398 | 0.70 |
| C400 | 0.011 | 0.144 | 0.23 |
| C331 | 0.075 | 0.134 | 0.21 |
| C422 | 0.11 | 0.234 | 0.35 |
| C511 | 0.12 | 0.171 | 0.19 |
| σ | 2.23 | 1.83 | 1.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baines, T.; Papageorgiou, G.; Hutter, O.S.; Bowen, L.; Durose, K.; Major, J.D. Self-Catalyzed CdTe Wires. Nanomaterials 2018, 8, 274. https://doi.org/10.3390/nano8050274
Baines T, Papageorgiou G, Hutter OS, Bowen L, Durose K, Major JD. Self-Catalyzed CdTe Wires. Nanomaterials. 2018; 8(5):274. https://doi.org/10.3390/nano8050274
Chicago/Turabian StyleBaines, Tom, Giorgos Papageorgiou, Oliver S. Hutter, Leon Bowen, Ken Durose, and Jonathan D. Major. 2018. "Self-Catalyzed CdTe Wires" Nanomaterials 8, no. 5: 274. https://doi.org/10.3390/nano8050274
APA StyleBaines, T., Papageorgiou, G., Hutter, O. S., Bowen, L., Durose, K., & Major, J. D. (2018). Self-Catalyzed CdTe Wires. Nanomaterials, 8(5), 274. https://doi.org/10.3390/nano8050274