Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Layer Separation of CAVD Leaflets is Confirmed by Histological Evaluation
2.2. Methacrylated Gelatin (GelMA) and Methacrylated Hyaluronic Acid (HAMA) Synthesis and Characterization
2.3. 3D-Bioprinting of Hybrid Hydrogels
2.3.1. Single-Layer Hydrogel Constructs
2.3.2. Dual-Layer Hydrogel Constructs
2.4. Mechanical Testing of CAVD Leaflets and Hydrogels
2.5. Human Aortic Valvular Interstitial Cell (VIC) Isolation, Culture, and Encapsulation in Hydrogels
2.6. Calcification and Apoptosis Assays
2.7. Analysis of VIC Remodeling Capability
2.8. Electron Microscopy
2.9. Statistical Methods
3. Results
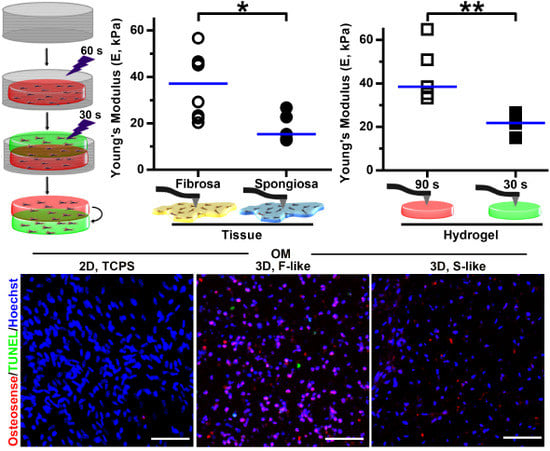
3.1. Leaflet Layer Separation of Human Aortic Valves by Microdissection
3.2. 3D-Bioprinting of Hybrid Hydrogel Constructs
3.3. Leaflet Layer Mechanical Properties Were Recapitulated in Hydrogels
3.4. VIC Encapsulation Affects Hydrogel Mechanics
3.5. Preferential Formation of Microcalcification in Fibrosa-Like Hydrogels
3.6. VIC Remodeling of Fibrosa-Like Hydrogels
3.7. Integration of Single-Layer Fibrosa-Like and Spongiosa-Like Hydrogels into a 3D-Bioprinted Dual-Layer Construct
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Messika-Zeitoun, D.; Bielak, L.F.; Peyser, P.A.; Sheedy, P.F.; Turner, S.T.; Nkomo, V.T.; Breen, J.F.; Maalouf, J.; Scott, C.; Tajik, A.J.; et al. Aortic valve calcification: Determinants and progression in the population. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Libby, P. A rock and a hard place: Chiseling away at the multiple mechanisms of aortic stenosis. Circulation 2017, 135, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Blaser, M.C.; Aikawa, E. Giving calcification its due: Recognition of a diverse disease: A first attempt to standardize the field. Circ. Res. 2017, 120, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Yabusaki, K.; Hutcheson, J.D.; Vyas, P.; Bertazzo, S.; Body, S.C.; Aikawa, M.; Aikawa, E. Quantification of calcified particles in human valve tissue reveals asymmetry of calcific aortic valve disease development. Front. Cardiovasc. Med. 2016, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.Y.; Chen, J.H.; Zhao, R.; Simmons, C.A. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, W.-L.; Sider, K.; Yip, C.Y.; Simmons, C.A. Beta-catenin mediates mechanically-regulated, tgf-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Yin, Z.; Hockaday Kang, L.; Magin, R.L.; Butcher, J.T. Active tissue stiffness modulation controls valve interstitial cell phenotype and osteogenic potential in 3d culture. Acta Biomater. 2016, 36, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Balguid, A.; Rubbens, M.P.; Mol, A.; Bank, R.A.; Bogers, A.J.; van Kats, J.P.; de Mol, B.A.; Baaijens, F.P.; Bouten, C.V. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets--relevance for tissue engineering. Tissue Eng. 2007, 13, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Stella, J.A.; Sacks, M.S. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J. Biomech. Eng. 2007, 129, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Vesely, I.; Noseworthy, R. Micromechanics of the fibrosa and the ventricularis in aortic valve leaflets. J. Biomech. 1992, 25, 101–113. [Google Scholar] [CrossRef]
- Hinderer, S.; Seifert, J.; Votteler, M.; Shen, N.; Rheinlaender, J.; Schaffer, T.E.; Schenke-Layland, K. Engineering of a bio-functionalized hybrid off-the-shelf heart valve. Biomaterials 2014, 35, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.C.; Wei, K.; Adams, R.L.; Zhou, Y.Q.; Caruso, L.L.; Mirzaei, Z.; Lam, A.; Tam, R.K.; Zhang, H.; Heximer, S.P.; et al. Deficiency of natriuretic peptide receptor 2 promotes bicuspid aortic valves, aortic valve disease, left ventricular dysfunction, and ascending aortic dilatations in mice. Circ. Res. 2018, 122, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Schlotter, F.; Halu, A.; Goto, S.; Blaser, M.C.; Body, S.C.; Lee, L.H.; Higashi, H.; DeLaughter, D.M.; Hutcheson, J.D.; Vyas, P.; et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 2018, 137. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.M.; Lawrence, R.L.; Anseth, K.S. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 2015, 49, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Camci-Unal, G.; Hutcheson, J.D.; Jung, S.M.; Schoen, F.J.; Kluin, J.; Aikawa, E.; Khademhosseini, A. Directing valvular interstitial cell myofibroblast-like differentiation in a hybrid hydrogel platform. Adv. Healthc. Mater. 2015, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Goettsch, C.; Hutcheson, J.D.; Camci-Unal, G.; Lax, L.; Scherer, K.; Body, S.; Schoen, F.J.; Kluin, J.; Khademhosseini, A.; et al. Simulation of early calcific aortic valve disease in a 3d platform: A role for myofibroblast differentiation. J. Mol. Cell. Cardiol. 2016, 94, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3d bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, C.F.; Wu, P.J.; Tibbitt, M.W.; van Mil, A.; Sluijter, J.P.; Langer, R.; Aikawa, E. In vitro 3d model and mirna drug delivery to target calcific aortic valve disease. Clin. Sci. 2017, 131, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.L.; Liss, H.; Raffaelli, S.; Humayun, A.; Khouri, K.S.; Coelho, P.G.; Witek, L. The technique for 3d printing patient-specific models for auricular reconstruction. J. Craniomaxillofac. Surg. 2017, 45, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Bhora, F.Y.; Lewis, E.E.; Rehmani, S.S.; Ayub, A.; Raad, W.; Al-Ayoubi, A.M.; Lebovics, R.S. Circumferential three-dimensional-printed tracheal grafts: Research model feasibility and early results. Ann. Thorac. Surg. 2017, 104, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, K.; Liu, S.; Zhou, X.; Rajagopal, V.; Meduri, C.; Kauten, J.R.; Chang, Y.H.; Wu, C.; Zhang, C.; et al. Quantitative prediction of paravalvular leak in transcatheter aortic valve replacement based on tissue-mimicking 3d printing. JACC. Cardiovasc. Imaging 2017, 10, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Schwarzer, N.; Sherratt, M.J.; Watson, R.E.; Graham, H.K.; Trafford, A.W.; Mummery, P.M.; Derby, B. Nanoindentation of histological specimens: Mapping the elastic properties of soft tissues. J. Mater. Res. 2009, 24, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Draper, E.R.; Adams, D.J.; Pfaff, H. Complex Shear Modulus of Hydrogels Using a Dynamic Nanoindentation Method; Springer International Publishing: Cham, Switzerland, 2016; pp. 141–145. [Google Scholar]
- Cohen, S.R.; Kalfon-Cohen, E. Dynamic nanoindentation by instrumented nanoindentation and force microscopy: A comparative review. Beilstein J. Nanotechnol. 2013, 4, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.P.C.; Zheng, Y.P. Estimation of young’s modulus and poisson’s ratio of soft tissue from indentation using two different-sized indentors: Finite element analysis of the finite deformation effect. Med. Biol. Eng. Comput. 2005, 43, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.S.; Sabbah, H.N.; Stein, P.D. Vibrational analysis of bioprosthetic heart valve leaflets using numerical models: Effects of leaflet stiffening, calcification, and perforation. Circ. Res. 1987, 61, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Riem Vis, P.W.; Bouten, C.V.; Sluijter, J.P.; Pasterkamp, G.; van Herwerden, L.A.; Kluin, J. Platelet-lysate as an autologous alternative for fetal bovine serum in cardiovascular tissue engineering. Tissue Eng. Part A 2010, 16, 1317–1327. [Google Scholar] [PubMed]
- Aper, S.J.; van Spreeuwel, A.C.; van Turnhout, M.C.; van der Linden, A.J.; Pieters, P.A.; van der Zon, N.L.; de la Rambelje, S.L.; Bouten, C.V.; Merkx, M. Colorful protein-based fluorescent probes for collagen imaging. PLoS ONE 2014, 9, e114983. [Google Scholar] [CrossRef] [PubMed]
- Thubrikar, M. The Aortic Valve; CRC Press, Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Schmidt, M.; Rodler, N.; Miesbauer, O.; Rojahn, M.; Vogel, T.; Dörfler, R.; Kucukpinar, E.; Langowski, H.-C. Adhesion and barrier performance of novel barrier adhesives used in multilayered high-barrier laminates. J. Adhes. Sci. Technol. 2012, 26, 2405–2436. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Sosnovik, D.; Lok, V.M.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 2007, 115, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Satta, J.; Oiva, J.; Salo, T.; Eriksen, H.; Ohtonen, P.; Biancari, F.; Juvonen, T.S.; Soini, Y. Evidence for an altered balance between matrix metalloproteinase-9 and its inhibitors in calcific aortic stenosis. Ann. Thorac. Surg. 2003, 76, 681–688. [Google Scholar] [CrossRef]
- Yip, C.Y.; Simmons, C.A. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc. Pathol. 2011, 20, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.H.; Kim, H.S.; Balachandran, K.; Weiler, M.; Haj-Ali, R.; Yoganathan, A.P. Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H395–H405. [Google Scholar] [CrossRef] [PubMed]
- Szeto, K.; Pastuszko, P.; del Alamo, J.C.; Lasheras, J.; Nigam, V. Bicuspid aortic valves experience increased strain as compared to tricuspid aortic valves. World J. Pediat. Congenit. Heart Surg. 2013, 4, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Sewell-Loftin, M.K.; Brown, C.B.; Baldwin, H.S.; Merryman, W.D. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 2012, 21, 513–520. [Google Scholar] [PubMed]
- Chen, J.H.; Simmons, C.A. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: Critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 2011, 108, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Sider, K.L.; Blaser, M.C.; Simmons, C.A. Animal models of calcific aortic valve disease. Int. J. Inflamm. 2011, 2011, 364310. [Google Scholar] [CrossRef] [PubMed]
- Sim, E.K.; Muskawad, S.; Lim, C.S.; Yeo, J.H.; Lim, K.H.; Grignani, R.T.; Durrani, A.; Lau, G.; Duran, C. Comparison of human and porcine aortic valves. Clin. Anat. 2003, 16, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Sider, K.L.; Zhu, C.; Kwong, A.V.; Mirzaei, Z.; de Lange, C.F.; Simmons, C.A. Evaluation of a porcine model of early aortic valve sclerosis. Cardiovasc. Pathol. 2014, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- van Geemen, D.; Soares, A.L.; Oomen, P.J.; Driessen-Mol, A.; Janssen-van den Broek, M.W.; van den Bogaerdt, A.J.; Bogers, A.J.; Goumans, M.J.; Baaijens, F.P.; Bouten, C.V. Age-dependent changes in geometry, tissue composition and mechanical properties of fetal to adult cryopreserved human heart valves. PLoS ONE 2016, 11, e0149020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschlager, M.; Kenner, L.; et al. Comparison of cancer cells in 2d vs 3d culture reveals differences in akt-mtor-s6k signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.M.; Payne, S.Z.; Anseth, K.S. Microarray analyses to quantify advantages of 2d and 3d hydrogel culture systems in maintaining the native valvular interstitial cell phenotype. Biomaterials 2016, 74, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Simard, L.; Cote, N.; Dagenais, F.; Mathieu, P.; Couture, C.; Trahan, S.; Bosse, Y.; Mohammadi, S.; Page, S.; Joubert, P.; et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: Is valvular fibrosis the explanation? Circ. Res. 2017, 120, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: Implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H756–H764. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Otto, I.A.; Breugem, C.C.; Malda, J.; Bredenoord, A.L. Ethical considerations in the translation of regenerative biofabrication technologies into clinic and society. Biofabrication 2016, 8, 042001. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Valk, D.C.; Van der Ven, C.F.T.; Blaser, M.C.; Grolman, J.M.; Wu, P.-J.; Fenton, O.S.; Lee, L.H.; Tibbitt, M.W.; Andresen, J.L.; Wen, J.R.; et al. Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics. Nanomaterials 2018, 8, 296. https://doi.org/10.3390/nano8050296
Van der Valk DC, Van der Ven CFT, Blaser MC, Grolman JM, Wu P-J, Fenton OS, Lee LH, Tibbitt MW, Andresen JL, Wen JR, et al. Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics. Nanomaterials. 2018; 8(5):296. https://doi.org/10.3390/nano8050296
Chicago/Turabian StyleVan der Valk, Dewy C., Casper F. T. Van der Ven, Mark C. Blaser, Joshua M. Grolman, Pin-Jou Wu, Owen S. Fenton, Lang H. Lee, Mark W. Tibbitt, Jason L. Andresen, Jennifer R. Wen, and et al. 2018. "Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics" Nanomaterials 8, no. 5: 296. https://doi.org/10.3390/nano8050296
APA StyleVan der Valk, D. C., Van der Ven, C. F. T., Blaser, M. C., Grolman, J. M., Wu, P. -J., Fenton, O. S., Lee, L. H., Tibbitt, M. W., Andresen, J. L., Wen, J. R., Ha, A. H., Buffolo, F., Van Mil, A., Bouten, C. V. C., Body, S. C., Mooney, D. J., Sluijter, J. P. G., Aikawa, M., Hjortnaes, J., ... Aikawa, E. (2018). Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics. Nanomaterials, 8(5), 296. https://doi.org/10.3390/nano8050296