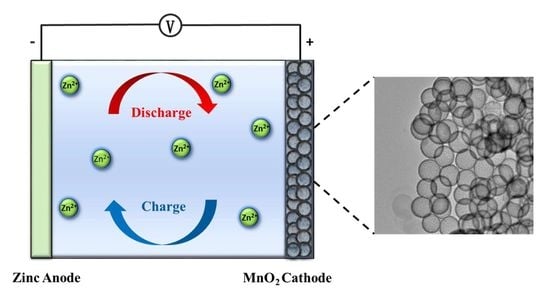
A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hollow Spherical MnO2 Particles
2.2. Cell Assembly and Test
2.3. Materials Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, Y.W.; Shao, Y.Y.; Raju, V.; Ji, X.L.; Mehdi, B.L.; Han, K.S.; Engelhard, M.H.; Li, G.S.; Browning, N.D.; Mueller, K.T.; et al. Molecular storage of Mg ions with vanadium oxide nanoclusters. Adv. Funct. Mater. 2016, 26, 3446–3453. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Chen, T.; Lv, H.; Zhu, G.; Ma, L.; Wang, C.; Jin, Z.; Liu, J. Emerging non-lithium ion batteries. Energy Storage Mater. 2016, 4, 103–129. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Lee, C.S.; Tang, Y.B. Low-cost metallic anode materials for high performance rechargeable batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Hertzberg, B.J.; Huang, A.; Hsieh, A.; Chamoun, M.; Davies, G.; Seo, J.K.; Zhong, Z.; Croft, M.; Erdonmez, C.; Meng, Y.S.; et al. Effect of multiple cation electrolyte mixtures on rechargeable Zn MnO2 alkaline battery. Chem. Mater. 2016, 28, 4536–4545. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Ort, V.D.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhang, X.Y.; Meng, Y.; Yu, M.H.; Yi, J.N.; Wu, Y.Q.; Lu, X.H.; Tong, Y.X. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. Aqueous rechargeable Zn-ion batteries: An imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J. Mater. Chem. A 2018, 6, 3850–3856. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.Y.; Liu, Y.C.; Zhao, Q.; Lei, K.X.; Chen, C.C.; Liu, X.S.; Chen, J. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-Ion battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Luo, L.L.; Zhong, L.; Chen, J.Z.; Li, B.; Wang, W.; Mao, S.X.; Wang, C.M.; Sprenkle, V.L.; Li, G.S.; et al. Highly reversible zinc-ion intercalation into chevrel phase MO6S8 nanocubes and applications for advanced zinc-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 13673–13677. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhu, T.; Wang, X.; Wei, X.; Yan, M.; Li, J.; Luo, W.; Yang, W.; Zhang, W.; Zhou, L.; et al. Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery. Nano Lett. 2018, 18, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zheng, S.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Advanced batteries based on manganese dioxide and its composites. Energy Storage Mater. 2018, 12, 284–309. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, Y.; Hobson, T.; Liu, J. Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett. 2010, 10, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.P.; Hu, Z.; Zhang, X.L.; Tao, Z.L.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Alfaruqi, M.H.; Song, J.; Kim, S.; Pham, D.T.; Jo, J.; Kim, S.; Mathew, V.; Baboo, J.P.; Xiu, Z.; et al. Carbon-coated manganese dioxide nanoparticles and their enhanced electrochemical properties for zinc-ion battery applications. J. Energy Chem. 2017, 26, 815–819. [Google Scholar] [CrossRef]
- Ko, J.S.; Sassin, M.B.; Parker, J.F.; Rolison, D.R.; Long, J.W. Combining battery-like and pseudocapacitive charge storage in 3D MnOx@Carbon electrode architectures for zinc-ion cells. Sustain. Energy Fuels 2018, 2, 626–636. [Google Scholar] [CrossRef]
- Xu, C.J.; Li, B.H.; Du, H.D.; Kang, F.Y. Energetic Zinc ion chemistry: The rechargeable Zinc ion battery. Angew. Chem.-Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cheng, F.Y.; Liu, J.X.; Wang, L.B.; Long, X.H.; Liu, X.S.; Li, F.J.; Chen, J. Rechargeable aqueous Zinc-Manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Duffort, V.; Mehdi, B.L.; Browning, N.D.; Nazar, L.F. Investigation of the mechanism of mg insertion in birnessite in nonaqueous and aqueous rechargeable mg-ion batteries. Chem. Mater. 2016, 28, 534–542. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, S.; Lee, S.; Salama, M.; Shterenberg, I.; Gofer, Y.; Kim, J.S.; Yang, E.; Park, C.S.; Kim, J.S.; et al. The high performance of crystal water containing manganese birnessite cathodes for magnesium batteries. Nano Lett. 2015, 15, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Han, S.D.; Kim, S.; Li, D.G.; Petkov, V.; Yoo, H.D.; Phillips, P.J.; Wang, H.; Kim, J.J.; More, K.L.; Key, B.; et al. Mechanism of Zn insertion into nanostructured delta-MnO2: A nonaqueous rechargeable Zn metal battery. Chem. Mater. 2017, 29, 4874–4884. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. A layered delta-MnO2 nanoflake cathode with high Zinc-storage capacities for eco-friendly battery applications. Electrochem. Commun. 2015, 60, 121–125. [Google Scholar] [CrossRef]
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.C.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, H.; Wu, H.B.; Lou, X.W. Complex hollow nanostructures: Synthesis and energy-related applications. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhuang, Z.C.; Zhao, H.H.; Lin, M.T.; Zhao, D.Y.; Mai, L.Q. Intricate hollow structures: Controlled synthesis and applications in energy storage and conversion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Birkner, N.; Navrotsky, A. Thermodynamics of manganese oxides: Sodium, potassium, and calcium birnessite and cryptomelane. Proc. Natl. Acad. Sci. USA 2017, 114, E1046–E1053. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries. Nanomaterials 2018, 8, 301. https://doi.org/10.3390/nano8050301
Guo X, Li J, Jin X, Han Y, Lin Y, Lei Z, Wang S, Qin L, Jiao S, Cao R. A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries. Nanomaterials. 2018; 8(5):301. https://doi.org/10.3390/nano8050301
Chicago/Turabian StyleGuo, Xiaotong, Jianming Li, Xu Jin, Yehu Han, Yue Lin, Zhanwu Lei, Shiyang Wang, Lianjie Qin, Shuhong Jiao, and Ruiguo Cao. 2018. "A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries" Nanomaterials 8, no. 5: 301. https://doi.org/10.3390/nano8050301
APA StyleGuo, X., Li, J., Jin, X., Han, Y., Lin, Y., Lei, Z., Wang, S., Qin, L., Jiao, S., & Cao, R. (2018). A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries. Nanomaterials, 8(5), 301. https://doi.org/10.3390/nano8050301