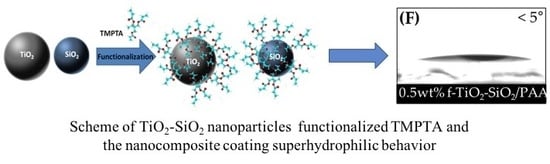
Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of TiO2–SiO2 Nanoparticles
2.3. Preparation of TiO2–SiO2 Embedded Nanocomposite Coatings
2.4. Characterization
3. Results
3.1. Functionalization of TiO2–SiO2 Nanoparticles
3.2. Surface Wettability of Nanocomposite Coatings
3.3. Transparency of Nanocomposite Coatings
3.4. Morphology and Roughness Analysis of f-TiO2–SiO2 Nanocomposite Coatings
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chatterjee, U.; Jewrajka, S.K.; Guha, S. Dispersion of functionalized silver nanoparticles in polymer matrices: Stability, characterization, and physical properties. Polym. Compos. 2009, 30, 827–834. [Google Scholar] [CrossRef]
- Peng, S.; Dang, B.; Zhou, Y.; Hu, J.; He, J. Functionalized TiO2 Nanoparticles Tune the Aggregation Structure and Trapping Property of Polyethylene Nanocomposites. J. Phys. Chem. C 2016, 120, 24754–24761. [Google Scholar] [CrossRef]
- Dhapte, V.; Kadam, S.; Pokharkar, V.; Khanna, P.K.; Dhapte, V. Versatile SiO2 Nanoparticles@Polymer Composites with Pragmatic Properties. ISRN Inorg. Chem. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Shen, J.; Yang, T.; Zhang, Q.; Zhou, B.; Deng, Z.; Fan, B.; Zhou, D.; Zhang, F. A novel route to control refrective index of sol-gel derived nano-porous silica films used as broadband antireflective coatings. Mater. Sci. Eng. 2000, B78, 135–139. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Murakami, T.; Fujishima, A. Sol-gel SiO2/TiO2 bilayers films with self cleanig and antireflection properties. Sol. Energy Mater. Sol. Cells 2008, 92, 1434–1438. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Yang, H.; Che, Q. Development of high dispersed TiO2 paste for transparent screen-printable self-cleaning coatings on glass. J. Nanopart. Res. 2013, 15, 1384. [Google Scholar] [CrossRef]
- Jesus, M.A.M.L.d.; Neto, J.T.d.S.; Timo, G.; Paiva, P.R.P.; Dantas, M.S.S.; Ferreira, A.d.M. Superhydrophilic self-cleaning surface based on TiO2 and TiO2/SiO2 composite for photovoltaic module cover glass. Appl. Adhes. Sci. 2015, 3, 5. [Google Scholar] [CrossRef]
- Alfieri, I.; Lorenzi, A.; Ranzenigo, L.; Lazzarini, L.; Predieri, G.; Lottici, P.P. Synthesis and characterization of photocatalytic hydrophobic hybrid TiO2-SiO2 coatings for building applications. Build. Environ. 2016, 111, 72–79. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Du, L.-C.; Zhang, Q.-L.; Zhang, L. A transient molecular probe for characterizing the surface properties of TiO2 nanoparticle in colloidal solution. Sci. Technol. Adv. Mater. 2005, 6, 867–872. [Google Scholar] [CrossRef]
- Zhang, M.; Breiner, T.; Mori, H.; Müller, A.H.E. Amphiphilic cylindrical brushes with poly(acrylic acid) core and poly(n-butyl acrylate) shell and narrow length distribution. Polymer 2003, 44, 1449–1458. [Google Scholar] [CrossRef]
- Guidotti, B.R.; Caseri, W.R.; Suter, U.W. Modification of SiO2 Surfaces by Reaction with Trialkoxymethanes and Triphenoxymethane. Langmuir 1996, 12, 4391–4394. [Google Scholar] [CrossRef]
- Ramírez-García, R.E.; Pérez-García, S.A.; González-Rodríguez, J.A.; Arroyo-Ortega, M.; Licea-Jiménez, L. Engineered TiO2 and SiO2-TiO2 films on silica-coated glass for increased thin film durability under abrasive conditions. Int. J. Appl. Ceram. Technol. 2017, 14, 39–49. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, A. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar]
- Villa, S.; Riani, P.; Locardi, F.; Canepa, F. Functionalization of Fe(3)O(4) NPs by Silanization: Use of Amine (APTES) and Thiol (MPTMS) Silanes and Their Physical Characterization. Materials 2016, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Li, J.R.; Jiang, L.; Chen, X.Y.; Li, J.R. Two-dimensional arrangement of octadecylamine-functionalized gold nanoparticles using the LB technique. Nanotechnology 2000, 11, 108. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shao, X. A Laser Fabrication of Magnetic Micromachines by Using Optimized Photosensitive Ferrofluids. J. Nanomater. 2016, 2016, 4. [Google Scholar] [CrossRef]
- Tamai, T.; Watanabe, M.; Ikeda, S.; Kobayashi, Y.; Fujiwara, Y.; Matsukawa, K. A Photocurable Pd Nanoparticle/Silica Nanoparticle/Acrylic Polymer Hybrid Layer for Direct Electroless Copper Deposition on a Polymer Substrate. J. Photopolym. Sci. Technol. 2012, 25, 141–146. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Matthew, L.Z.M.; Wei-Cheng, W.; Nathan, A.O.; Michael, G.; Baohua, M.; Per-Anders, G.; Peter, Y.; Jinghua, G.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef] [PubMed]
- Khung, Y.L.; Ngalim, S.H.; Scaccabarozz, A.; Narducci, D. Formation of stable Si–O–C submonolayers on hydrogenterminated silicon(111) under low-temperature conditions. Beilstein J. Nanotechnol. 2015, 6, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Shirke, B.S.; Korake, P.V.; Hankare, P.P.; Bamane, S.R.; Garadkar, K.M. Synthesis and characterization of pure anatase TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2011, 22, 821–824. [Google Scholar] [CrossRef]
- Valappil Swapna, M.; Haridas, K.R. Sonochemical Synthesis and Morphological Study of Nanocrystalline Rutile TiO2. Chemist 2015, 88, 1–6. [Google Scholar]
- Wang, W.; Martin, J.C.; Huang, R.; Huang, W.; Liu, A.; Han, A.; Sun, L. Synthesis of silicon complexes from rice husk derived silica nanoparticles. RSC Adv. 2012, 2, 9036–9041. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Wang, M.; Cai, J.; Xu, J.; Xia, C. Facile preparation of highly-dispersed cobalt-silicon mixed oxide nanosphere and its catalytic application in cyclohexane selective oxidation. Nanoscale Res. Lett. 2011, 6, 586. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Farady Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Miljevic, B.; Hedayat, F.; Stevanovic, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D. To Sonicate or Not to Sonicate PM Filters: Reactive Oxygen Species Generation Upon Ultrasonic Irradiation. Aerosol Sci. Technol. 2014, 48, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.J.; Saranya, S.; Sharma, P.; Tamuli, R.; Bora, U. Gold nanoparticles: Sonocatalytic synthesis using ethanolic extract of Andrographis paniculata and functionalization with polycaprolactone-gelatin composites. Front. Mater. Sci. 2012, 6, 236–249. [Google Scholar] [CrossRef]
- Hair, M.-L. Hydroxyl Groups on Silica Surface. J. Non-Cristal. Solids 1975, 19, 299–309. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Kohtani, S.; Miyabe, H. Titanium Dioxide -Induced Photocatalytic Reduction for Organic Synthesis. In Titanium Dioxide: Chemical Properties, Applications and Environmental Effects; Brown, J., Ed.; Nova Science: Hauppauge, NY, USA, 2014; pp. 157–176. [Google Scholar]
- Harada, Y.; Ogawa, K.; Irie, Y.; Endo, H.; Feril, L.B.; Uemura, T.; Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J. Control. Release 2011, 149, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Ogino, C.; Dadjour, M.F.; Murata, T. Sonocatalytic degradation of methylene blue with TiO2 pellets in water. Ultrason. Sonochem. 2007, 14, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef] [PubMed]













| Nanocomposite | Nanoparticle | wt % | Nomenclature |
|---|---|---|---|
| TiO2–SiO2/PAA | Non-functionalized | 0.1 | 0.1 wt % TiO2–SiO2/PAA |
| 0.5 | 0.5 wt % TiO2–SiO2/PAA | ||
| f-TiO2–SiO2/PAA | Functionalized | 0.1 | 0.1 wt % f-TiO2–SiO2/PAA |
| 0.5 | 0.5 wt % f-TiO2–SiO2 /PAA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Velázquez, A.R.; Velasco-Soto, M.A.; Pérez-García, S.A.; Licea-Jiménez, L. Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials 2018, 8, 369. https://doi.org/10.3390/nano8060369
Vázquez-Velázquez AR, Velasco-Soto MA, Pérez-García SA, Licea-Jiménez L. Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials. 2018; 8(6):369. https://doi.org/10.3390/nano8060369
Chicago/Turabian StyleVázquez-Velázquez, Arturo Román, Miguel Angel Velasco-Soto, Sergio Alfonso Pérez-García, and Liliana Licea-Jiménez. 2018. "Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties" Nanomaterials 8, no. 6: 369. https://doi.org/10.3390/nano8060369
APA StyleVázquez-Velázquez, A. R., Velasco-Soto, M. A., Pérez-García, S. A., & Licea-Jiménez, L. (2018). Functionalization Effect on Polymer Nanocomposite Coatings Based on TiO2–SiO2 Nanoparticles with Superhydrophilic Properties. Nanomaterials, 8(6), 369. https://doi.org/10.3390/nano8060369